Intracerebroventricular anaerobic dopamine in Parkinson's disease with L-dopa-related complications: a phase 1/2 randomized-controlled trial
- PMID: 39775041
- PMCID: PMC11922744
- DOI: 10.1038/s41591-024-03428-2
Intracerebroventricular anaerobic dopamine in Parkinson's disease with L-dopa-related complications: a phase 1/2 randomized-controlled trial
Abstract
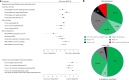
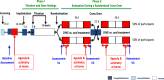
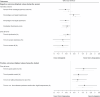
Continuous compensation for cerebral dopamine deficiency represents an ideal treatment for Parkinson's disease. Dopamine does not cross the digestive and blood-brain barriers and is rapidly oxidized. The new concept is the intracerebroventricular administration of anaerobic dopamine (A-dopamine) using an abdominal pump connected to a subcutaneous catheter implanted in the third ventricle, near the striatum. An open-label phase 1 study showed no serious adverse reactions induced by A-dopamine in 12 patients. A randomized, controlled, open-label, crossover phase 2 study of 1 month of A-dopamine versus 1 month of optimized oral antiparkinsonian therapy was conducted in 9 patients. The primary endpoint, a blinded assessment of the percentage over target (that is, time with dyskinesia or bradykinesia), recorded by home actimetry using a wristwatch, was significantly reduced on A-dopamine compared with that on oral treatment alone (P = 0.027), with a median within-patient difference of -10.4 (Hedge g = -0.62 (95% confidence interval: -1.43, -0.08)). Home diaries were also significantly improved. These initial data on the feasibility, safety and effects of this new device-assisted therapy suggest validation by a large randomized double-blind trial. ClinicalTrials.gov registration: NCT04332276 .
© 2025. The Author(s).
Conflict of interest statement
Competing interests: D.D., C.M., J.C.D. and M.F. have an equity stake in InBrain Pharma. A.D. and M.F. are employees of InBrain Pharma. C.M. has received grants from the France Parkinson charity and honoraria from Orkyn, Apopharma and Boston Scientific for consultancy and lectures on Parkinson’s disease at symposia. C.M. is CMO of Feetme and holds stakes in InBrain Pharma and InVenis Biotherapies. P.O. is the director of the pharmaceutical department of the University Hospital of Lille and leads the research group on injectable forms and associated technologies. As such, P.O. has signed contracts with many pharmaceutical companies. All contracts are signed by delegations from the University of Lille or CHU Lille. There are no personal contracts. D.D. has received PHRC grants from the French Ministry of Health (PHRC and ANR), European grants (H2020 and Coen) and research funding from the ARSLA charity, France; Parkinson charity; Fondation Credit Agricole, and Fondation de France. He has served on advisory boards, served as a consultant and given lectures for pharmaceutical companies such as Abbvie, Alterity, Orkyn, Air Liquide, Elivie, Homeperf, Apopharma, Lundbeck, Everpharma, Medtronic, Boston Scientific, Everpharma, UCB Pharma, EISAI, Servier, PTC Therapeutics, Orion, AB Science, Alzprotect, Cajal Neuroscience and Cure Parkinson Trust. He holds stakes in InBrain Pharma and InVenis Biotherapies. G.T., N.R., C.B., D.L., N.C., B.P., F.M., B.G., K.D., L.C., A.S.R., D.D. and J.C.D. have nothing to declare.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

