Neoadjuvant anti-PD-1 alone or in combination with anti-TIGIT or an oncolytic virus in resectable stage IIIB-D melanoma: a phase 1/2 trial
- PMID: 39775043
- PMCID: PMC11750705
- DOI: 10.1038/s41591-024-03411-x
Neoadjuvant anti-PD-1 alone or in combination with anti-TIGIT or an oncolytic virus in resectable stage IIIB-D melanoma: a phase 1/2 trial
Abstract
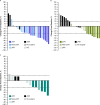
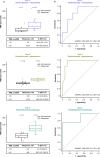
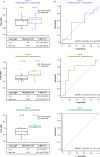
Neoadjuvant immunotherapies have shown antitumor activity in melanoma. Substudy 02C of the global, rolling-arm, phase 1/2, adaptive-design KEYMAKER-U02 trial is evaluating neoadjuvant pembrolizumab (anti-PD-1) alone or in combination, followed by adjuvant pembrolizumab, for stage IIIB-D melanoma. Here we report results from the first three arms: pembrolizumab plus vibostolimab (anti-TIGIT), pembrolizumab plus gebasaxturev (coxsackievirus A21) and pembrolizumab monotherapy. Pathologic complete responses occurred in 10 of 26 patients (38%) with pembrolizumab plus vibostolimab, 7 of 25 (28%) with pembrolizumab plus gebasaxturev and 6 of 15 (40%) with pembrolizumab monotherapy. Major pathologic responses occurred in 13 (50%), 10 (40%) and 7 (47%) patients, respectively. Safety was manageable. Treatment-related adverse events occurred in 24 of 26 patients (92%) with pembrolizumab plus vibostolimab, 21 of 25 (84%) with pembrolizumab plus gebasaxturev and 12 of 15 (80%) with pembrolizumab monotherapy; grade 3 or 4 treatment-related adverse events occurred in 2 (8%), 7 (28%) and 1 (7%) patient in each arm, respectively. No deaths due to adverse events occurred. Exploratory objective responses per RECIST v1.1 were observed in 13 (50%), 8 (32%) and 4 (27%) patients, in each arm, respectively. In a post hoc analysis, scores for tumor mutational burden and an 18-gene T cell-inflamed gene expression profile were generally higher in patients with major pathologic response. Longer follow-up will provide insight into the incremental benefit of combining neoadjuvant pembrolizumab with other therapies in stage IIIB-D melanoma. ClinicalTrials.gov registration: NCT04303169 .
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare the following competing interests: R.D. reports consulting fees and payment or honoraria for lectures, presentations, speakers’ bureaus, paper writing or educational events from Amgen, Bristol Myers Squibb, MSD, Novartis, Pierre Fabre, Roche, Sun Pharma, Takeda, Sanofi, Caralym, Second-Genome, Regeneron, Alligator, T3 Pharma, MaxiVAX SA, Pfizer, Simcere and touchIME. C.R. reports consulting fees from BMS, Roche, Pierre Fabre, Novartis, Sanofi, Pfizer, MSD, Sun Pharma and AstraZeneca and participation on a Data Safety Monitoring Board or Advisory Board for BMS, Roche, Pierre Fabre, Novartis, Sanofi, Pfizer, MSD, Sun Pharma and AstraZeneca. R.A.S. reports consulting fees from SkylineDx BV, F. Hoffman-La Roche Ltd, MetaOptima Technology Inc., Evaxion, Provectus Biopharmaceuticals Australia, Qbiotics, Novartis, Merck Sharp & Dohme, NeraCare, AMGEN Inc, Bristol Myers Squibb, Myriad Genetics, GlaxoSmithKline and IO Biotech ApS. J.M.T. reports grants or contracts to their institution from BMS; consulting fees from Merck & Co, BMS, AstraZeneca, Regeneron, Roche, Elephas, Lunaphore and Akoya Biosciences; a patent planned for machine learning for irPRC; and a Task Force role with the Society for Immunotherapy of Cancer. A.M.M. reports consulting fees from BMS, MSD, Novartis, Pierre Fabre, Roche and Qbiotics. M.T.T. reports support for the present paper to their institution from Merck and payment or honoraria and support for attending the Clinical Care Options, AACR 2023 companion course. A.H. has no conflicts of interest to declare. J.-J.G. reports consulting fees from Novartis and BMS, and participation on a Data Safety Monitoring Board or Advisory Board for Novartis, BMS, MSD, Philogen, Pierre Fabre, Sanofi, Roche and Amgen. D.C.P. has no conflicts of interest to declare. C.L. reports participating on an Advisory Board for MSD, BMS, Pierre Fabre, Novartis, Regenron and Seacraft; and travel expenses from BMS. M.A.K. reports consulting fees from MSD Australia, BMS Australia and Moderna; payment or honoraria for lectures, presentations, speakers’ bureaus, paper writing or educational events from MSD Australia and Moderna; and support for attending meetings and/or travel from Moderna and Pierre Fabre. J.C. has no conflicts of interest to declare. G.B.-S. reports payment or honoraria for lectures, presentations, speakers’ bureaus, paper writing or educational events from BMS, MSD, Merck, Novartis, Medison and Sanofi, and a role as the chairman of the Israeli Society of Clinical Oncology & Radiation Therapy. I.M. reports payment or honoraria for lectures, presentations, speakers’ bureaus, paper writing or educational events from BMS and Immunocore, and stock or stock options in Immunocore, Delcath and In8 Bio Iovance Ideaya. R.S.-F. reports medical writing support for the present paper; grants or contracts to their institution from MSD; payment or honoraria for lectures, presentations, speakers’ bureaus, paper writing or educational events from MSD, BMS, Novartis, Sanofi, Roche, AstraZeneca, Medison and Neopharm; and participation on an advisory board for MSD, Novartis and Clovis Oncology. N.M. reports grants or contracts from BMS, Novartis, Merck, MSD and Pierre Fabre; payment or honoraria for lectures, presentations, speakers’ bureaus, paper writing or educational events from BMS; payment for expert testimony from BMS, Pierre Fabre, MSD and Novartis; and support for attending meetings and/or travel from Pierre Fabre, Janssen, BMS and AbbVie. A.L.W. is an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and owns stock in Merck & Co., Inc., Rahway, NJ, USA, and Organon. Y.R., M.F.-K. and C.K. are employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and own stock in Merck & Co., Inc., Rahway, NJ, USA. G.V.L. reports consulting fees from and participation on a Data Safety Monitoring Board or Advisory Board for Agenus Inc, Amgen Inc, Array BioPharma, AstraZeneca, Bayer Health Care, BioNTech, Boehringer Ingelheim International GmbH, Bristol Myers Squibb, Evaxion Biotech A/S, Hexal AG (Sandoz Company), Highlight Therapeutics S.L., IO Biotech, Immunocore Ireland Limited, Innovent Biologics USA Inc, Merck Sharp & Dohme (Australia) Pty Limited, Novartis Pharma AG, PHMR Ltd, Pierre Fabre, Qbiotics Group Limited, Regeneron Pharmaceuticals, Scancell Limited and SkylineDX B.V.; and payment or honoraria for lectures, presentations, speakers’ bureaus, paper writing or educational events from Bristol Myers Squibb and Pierre Fabre.
Figures





References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

