Intracerebroventricular B7-H3-targeting CAR T cells for diffuse intrinsic pontine glioma: a phase 1 trial
- PMID: 39775044
- PMCID: PMC11922736
- DOI: 10.1038/s41591-024-03451-3
Intracerebroventricular B7-H3-targeting CAR T cells for diffuse intrinsic pontine glioma: a phase 1 trial
Abstract
Diffuse intrinsic pontine glioma (DIPG) is a fatal central nervous system (CNS) tumor that confers a median survival of 11 months. As B7-H3 is expressed on pediatric CNS tumors, we conducted BrainChild-03, a single-center, dose-escalation phase 1 clinical trial of repetitive intracerebroventricular (ICV) dosing of B7-H3-targeting chimeric antigen receptor T cells (B7-H3 CAR T cells) for children with recurrent or refractory CNS tumors and DIPG. Here we report results from Arm C, restricted to patients with DIPG. The primary objectives were to assess feasibility and tolerability, which were both met. Secondary objectives included assessments of CAR T cell distribution and survival. A total of 23 patients with DIPG enrolled, and 21 were treated with repeated doses of ICV B7-H3 CAR T cells using intra-patient dose-escalation regimens without previous lymphodepletion. Concurrent tumor-directed therapy, including re-irradiation, was not allowed while on protocol therapy. We delivered a total of 253 ICV doses and established the highest planned dose regimen, DR4, which escalated up to 10 × 107 cells per dose, as the maximally tolerated dose regimen. Common adverse events included headache, fatigue and fever. There was one dose-limiting toxicity (intratumoral hemorrhage) during DR2. For all treated patients (n = 21), the median survival from their initial CAR T cell infusion was 10.7 months and the median survival from diagnosis was 19.8 months with 3 patients still alive at 44, 45 and 52 months from diagnosis. Ultimately, this completed first-in-human trial shows that repetitive ICV dosing of B7-H3 CAR T cells in pediatric and young adult patients with DIPG is tolerable, including multiyear repeated dosing, and may have clinical efficacy that warrants further investigation on a multisite phase 2 trial. ClinicalTrials.gov registration: NCT04185038 .
© 2025. The Author(s).
Conflict of interest statement
Competing interests: N.A.V. holds equity in and serves as the Scientific Advisory Board Chair for BrainChild Bio, Inc. J.A.G. holds equity in BrainChild Bio, Inc. R.J.O. receives research support from Lentigen Technology, a Miltenyi Biotec company, and is a consultant for Umoja Biopharma. R.A.G. is an inventor and receives royalties on patents related to CAR T cell technologies that are licensed to Juno Therapeutics, a Bristol Myers Squibb company, and serves as a consultant to Moonlight Bio. M.C.J. holds equity in and is the Chief Scientific Officer of BrainChild Bio, Inc. M.C.J. holds equity in, is a Board Observer for and serves as a member of the Joint Steering Committee of Umoja Biopharma, Inc. N.A.V., J.A.G., J.B.F., J.R.P. and M.C.J. are inventors on issued and pending patents related to CAR T cell therapies. The other authors declare no competing interests.
Figures
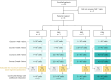
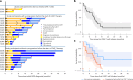


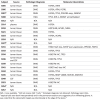

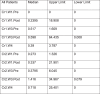

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

