Low-dimensional controllability of brain networks
- PMID: 39775065
- PMCID: PMC11706394
- DOI: 10.1371/journal.pcbi.1012691
Low-dimensional controllability of brain networks
Erratum in
-
Correction: Low-dimensional controllability of brain networks.PLoS Comput Biol. 2025 Oct 21;21(10):e1013601. doi: 10.1371/journal.pcbi.1013601. eCollection 2025 Oct. PLoS Comput Biol. 2025. PMID: 41118337 Free PMC article.
Abstract
Identifying the driver nodes of a network has crucial implications in biological systems from unveiling causal interactions to informing effective intervention strategies. Despite recent advances in network control theory, results remain inaccurate as the number of drivers becomes too small compared to the network size, thus limiting the concrete usability in many real-life applications. To overcome this issue, we introduced a framework that integrates principles from spectral graph theory and output controllability to project the network state into a smaller topological space formed by the Laplacian network structure. Through extensive simulations on synthetic and real networks, we showed that a relatively low number of projected components can significantly improve the control accuracy. By introducing a new low-dimensional controllability metric we experimentally validated our method on N = 6134 human connectomes obtained from the UK-biobank cohort. Results revealed previously unappreciated influential brain regions, enabled to draw directed maps between differently specialized cerebral systems, and yielded new insights into hemispheric lateralization. Taken together, our results offered a theoretically grounded solution to deal with network controllability and provided insights into the causal interactions of the human brain.
Copyright: © 2025 Ben Messaoud et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



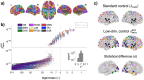


References
-
- Liu YY, Barabási AL. Control Principles of Complex Networks. Rev Mod Phys [Internet]. 2016. Sep 6 [cited 2017 May 16];88(3). Available from: http://arxiv.org/abs/1508.05384
-
- Pasqualetti F, Zampieri S, Bullo F. Controllability Metrics, Limitations and Algorithms for Complex Networks. IEEE Trans Control Netw Syst. 2014. Mar;1(1):40–52.
MeSH terms
LinkOut - more resources
Full Text Sources

