Early therapy initiation is crucial in chronic inflammatory demyelinating polyneuropathy: prospective multimodal data from the German INHIBIT registry
- PMID: 39775066
- PMCID: PMC11706869
- DOI: 10.1007/s00415-024-12860-w
Early therapy initiation is crucial in chronic inflammatory demyelinating polyneuropathy: prospective multimodal data from the German INHIBIT registry
Abstract
Background: Diagnosing chronic inflammatory demyelinating polyneuropathy (CIDP) can be challenging, leading to delays in initiating therapy. As disability in CIDP is mainly dependent on axonal damage, the impact of delayed immunotherapy remains unclear. We multimodally investigated the clinical outcomes of patients with early CIDP regarding different treatment strategies and time points.
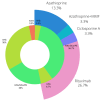
Methods: Patients with CIDP diagnosis within 1 year before study inclusion were systematically selected from the prospective Immune-mediated Neuropathies Biobank (INHIBIT) registry. Clinical and therapeutic data, and findings from nerve conduction study (NCS), and nerve and muscle ultrasound were correlated at inclusion and 12 months later. The patient outcomes were compared between immunotherapies. The effect of timing immunotherapy on clinical outcomes was determined using regression analysis.
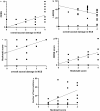
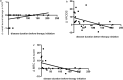
Results: In total, 30 patients were included (time from diagnosis to inclusion 22 ± 19 weeks). Low amplitudes of compound muscle potential were significantly associated with pathological spontaneous activity (PSA, r = 0.467) and correlated with the Heckmatt scale (rSp = 0.391). All three parameters were significantly associated with higher overall disability sum scores (NCS score rSp = 0.581, PSA r = 0.385, Heckmatt scale rSp = 0.472). The delays in initiating therapy resulted in progression of axonal damage (rSp = 0.467) and disability (R2 = 0.200). The combination of first-line therapies led to reduced disability progression (r = 0.773), while second-line therapies resulted in improved overall axonal damage (r = 0.467).
Conclusions: Axonal damage occurs early and is the main cause of clinical disabilities. Prompt initiation of therapy is crucial to prevent axonal damage and thereby disability progression. A comprehensive therapeutic approach, including a combination of first- or second-line therapies, may improve long-term outcomes.
Keywords: Axonal damage; CIDP; Muscle ultrasound; Therapy.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflicts of interest: Aurelian Schumacher has none related to this manuscript. Alina Hieke has none related to this manuscript. Marie Spenner has none related to this manuscript. Fynn Schmitz has none related to this manuscript. Melissa Sgodzai has none related to this manuscript. Rafael Klimas received research funding from The LFB Group France and Ruhr-University, Bochum and travel funding from Grifols and Takeda; not related to this work. Jil Brünger has none related to this manuscript. Sophie Huckemann has none related to this manuscript. Jeremias Motte: received travel grants and speaker honoraria from Alnylam Pharmaceuticals, Biogen idec, Bristol Myers Squibb, Novartis AG, Teva, Kyverna therapeutics and Eisai GmbH, his research is funded by Klaus Tschira Foundation and Ruhr-University, Bochum (FoRUM-program); Hertie foundation; Deutsche Forschungsgemeinschaft (DFG), Deutsche Multiple Sklerose Gesellschaft (DMSG), Biogen idec; Novartis, kyverna Therapeutics; none related to this work. Anna Lena Fisse received research funding by Georgius Agricola Stiftung Ruhr and Ruhr-University, Bochum (FoRUM-program), received honoraria and travel grants from Novartis AG, Sanofi and Eisai GmbH, none related to this work. Owns shares of Fresenius SE & Co., Gilead Sciences, Medtronic PLC and Novartis AG. None related to this work. Ralf Gold serves on scientific advisory boards for Teva Pharmaceutical Industries Ltd., Biogen Idec, Bayer Schering Pharma, and Novartis; has received speaker honoraria from Biogen Idec, Teva Pharmaceutical Industries Ltd., Bayer Schering Pharma, and Novartis; serves as editor for Therapeutic Advances in Neurological Diseases and on the editorial boards of Experimental Neurology and the Journal of Neuroimmunology; and receives research support from Teva Pharmaceutical Industries Ltd., Biogen Idec, Bayer Schering Pharma, Genzyme, Merck Serono, and Novartis, none related to this manuscript. Kalliopi Pitarokoili received travel funding and speaker honoraria from Biogen Idec, Novartis and Bayer Schering Pharma and funding from the Ruhr-University, Bochum (FORUM-Program), none related to this work. Thomas Grüter received travel funding and speaker honoraria from CSL Behring, Sanofi, Novartis, Biogen Idec, Bristol Myers Squibb, and Viatris, none related to this work. Ethical approval: The study was conducted in accordance with the Declaration of Helsinki of 1975 and approved by the local ethics committee of the Ruhr University Bochum (20-6827).
Figures
References
-
- Van den Bergh PYK, van Doorn PA, Hadden RDM, Avau B, Vankrunkelsven P, Allen JA et al (2021) European Academy of Neurology/Peripheral Nerve Society guideline on diagnosis and treatment of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force—second revision. Eur J Neurol 28:3556–3583. 10.1111/ene.14959 - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

