Kilohertz electrical stimulation evokes robust cellular responses like conventional frequencies but distinct population dynamics
- PMID: 39775118
- PMCID: PMC11706954
- DOI: 10.1038/s42003-024-07447-0
Kilohertz electrical stimulation evokes robust cellular responses like conventional frequencies but distinct population dynamics
Abstract
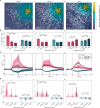
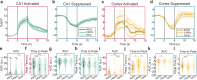
Intracranial electrical kilohertz stimulation has recently been shown to achieve similar therapeutic benefit as conventional frequencies around 140 Hz. However, it is unknown how kilohertz stimulation influences neural activity in the mammalian brain. Using cellular calcium imaging in awake mice, we demonstrate that intracranial stimulation at 1 kHz evokes robust responses in many individual neurons, comparable to those induced by conventional 40 and 140 Hz stimulation in both the hippocampus and sensorimotor cortex. The evoked responses at the single-cell level are shaped by prominent network inhibition and critically depend on brain region. At the network level, all frequencies lead to pronounced population suppression except 1 kHz in the cortex, which evokes balanced excitatory and inhibitory population effects. Thus, kilohertz stimulation robustly modulates neural activity at both the single-neuron and population network levels through mechanisms distinct from conventional frequency stimulation, highlighting the clinical potential of intracranial kilohertz neuromodulation.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures






Similar articles
-
High frequency electrical stimulation entrains fast spiking interneurons and bidirectionally modulates information processing.bioRxiv [Preprint]. 2025 Jul 4:2025.07.01.662561. doi: 10.1101/2025.07.01.662561. bioRxiv. 2025. PMID: 40631240 Free PMC article. Preprint.
-
Transcranial ultrasound stimulation modulates neuronal membrane potentials across broad timescales in the awake mammalian brain.bioRxiv [Preprint]. 2025 Jun 12:2025.06.09.658675. doi: 10.1101/2025.06.09.658675. bioRxiv. 2025. PMID: 40661565 Free PMC article. Preprint.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
Cited by
-
Human Applications of Transcranial Temporal Interference Stimulation: A Systematic Review.medRxiv [Preprint]. 2025 Jun 23:2025.05.16.25327804. doi: 10.1101/2025.05.16.25327804. medRxiv. 2025. PMID: 40463528 Free PMC article. Preprint.
References
-
- Alector Inc. A Phase 2 Randomized, Double-Blind, Placebo-Controlled, Multicenter Study to Evaluate the Efficacy and Safety of AL002 in Participants With Early Alzheimer’s Disease. https://clinicaltrials.gov/ct2/show/NCT04592874 (2021).
-
- Li, M. C. H. & Cook, M. J. Deep brain stimulation for drug-resistant epilepsy. Epilepsia59, 273–290 (2018). - PubMed
MeSH terms
Substances
Grants and funding
- 1R01NS115797/Foundation for the National Institutes of Health (Foundation for the National Institutes of Health, Inc.)
- RF1 NS129520/NS/NINDS NIH HHS/United States
- F31MH123008/Foundation for the National Institutes of Health (Foundation for the National Institutes of Health, Inc.)
- 5T32GM145455/Foundation for the National Institutes of Health (Foundation for the National Institutes of Health, Inc.)
- R01 NS115797/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources

