Histopathological effects of hypervitaminosis-D and the protective role of fetuin-A in renal, hepatic, and cardiac tissues in a murine model
- PMID: 39775139
- PMCID: PMC11706989
- DOI: 10.1038/s41598-025-85200-1
Histopathological effects of hypervitaminosis-D and the protective role of fetuin-A in renal, hepatic, and cardiac tissues in a murine model
Abstract
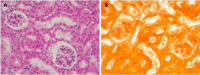
Hypervitaminosis D leads to toxic effects, including hypercalcemia, which can cause severe damage to various organs. Fetuin-A, a glycoprotein with anti-inflammatory properties, may protect tissues from such damage. This study explores the role of Fetuin-A in mitigating hypervitaminosis D-induced damage in renal, hepatic, and cardiac tissues. The objectives of this study were to: (1) Assess the extent of tissue damage from high-dose vitamin D in a murine model by examining the histopathological changes in liver, kidney and heart. (2) Investigate Fetuin-A's protective effect against this damage. Thirty-six albino rats were divided into four groups: (1) control, (2) vitamin D toxicity, (3) Fetuin-A + vitamin D, and (4) Fetuin-A only. Vitamin D was administered subcutaneously at 250 μg/20 g/day for 3 days. Fetuin-A was given at 100 μl/20 g, starting 7 days before vitamin D treatment. Histopathological analysis of liver, kidney, and heart tissues was performed using H&E and Alizarin Red staining and findings were analysed statistically. Vitamin D toxicity caused significant tissue damage, including apoptosis, inflammation, and calcification in the liver, kidneys, and heart. Pre-treatment with Fetuin-A reduced calcification and inflammation, preserving tissue architecture. Fetuin-A-only rats showed no damage or calcification. Fetuin-A provided statistically significant protection against vitamin D-induced damage, reducing oxidative stress and calcification in affected organs. These findings suggest Fetuin-A could be a potential therapeutic agent for hypervitaminosis D.
Keywords: Calcification; Fetuin-A; Hypervitaminosis D; Tissue damage; Vitamin D toxicity.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics approval: This project was approved by Institutional Review Board in Dubai Medical College for Girls and it was in compliance with the regulations of ethical treatment of laboratory animals. (REC AY22-23-F-01).
Figures











Similar articles
-
Intraperitoneal administration of fetuin-A attenuates D-galactosamine/lipopolysaccharide-induced liver failure in mouse.Dig Dis Sci. 2014 Aug;59(8):1789-97. doi: 10.1007/s10620-014-3071-0. Epub 2014 Mar 7. Dig Dis Sci. 2014. PMID: 24604240 Free PMC article.
-
Microvasculopathy and soft tissue calcification in mice are governed by fetuin-A, magnesium and pyrophosphate.PLoS One. 2020 Feb 19;15(2):e0228938. doi: 10.1371/journal.pone.0228938. eCollection 2020. PLoS One. 2020. PMID: 32074140 Free PMC article.
-
Fetuin-A protects against atherosclerotic calcification in CKD.J Am Soc Nephrol. 2009 Jun;20(6):1264-74. doi: 10.1681/ASN.2008060572. Epub 2009 Apr 23. J Am Soc Nephrol. 2009. PMID: 19389852 Free PMC article.
-
Tissue chaperoning-the expanded functions of fetuin-A beyond inhibition of systemic calcification.Pflugers Arch. 2022 Aug;474(8):949-962. doi: 10.1007/s00424-022-02688-6. Epub 2022 Apr 11. Pflugers Arch. 2022. PMID: 35403906 Free PMC article. Review.
-
The role of fetuin-A in physiological and pathological mineralization.Calcif Tissue Int. 2013 Oct;93(4):355-64. doi: 10.1007/s00223-012-9690-6. Epub 2013 Jan 1. Calcif Tissue Int. 2013. PMID: 23277412 Review.
Cited by
-
Evaluating Vitamin D Deficiency and Toxicity in Indian Children: A Retrospective Study.Cureus. 2025 Apr 20;17(4):e82647. doi: 10.7759/cureus.82647. eCollection 2025 Apr. Cureus. 2025. PMID: 40395263 Free PMC article.
References
-
- Kaur, P., Mishra, S. & Mithal, A. Vitamin d toxicity resulting from overzealous correction of vitamin d deficiency. Clin. Endocrinol.83(3), 327–331. 10.1111/cen.12836 (2015). - PubMed
-
- Silva, C. et al. Vitamin d toxicity from an unusual and unexpected source: a report of 2 cases. Hormone Res. Paediatr.96(3), 332–340. 10.1159/000526755 (2022). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

