FUT10 and FUT11 are protein O-fucosyltransferases that modify protein EMI domains
- PMID: 39775168
- PMCID: PMC11949838
- DOI: 10.1038/s41589-024-01815-x
FUT10 and FUT11 are protein O-fucosyltransferases that modify protein EMI domains
Abstract
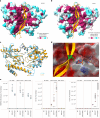
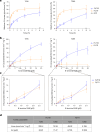
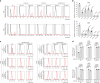
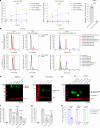
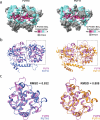
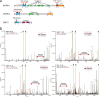
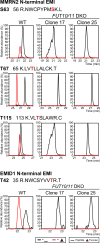
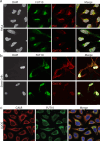
O-Fucosylation plays crucial roles in various essential biological events. Alongside the well-established O-fucosylation of epidermal growth factor-like repeats by protein O-fucosyltransferase 1 (POFUT1) and thrombospondin type 1 repeats by POFUT2, we recently identified a type of O-fucosylation on the elastin microfibril interface (EMI) domain of Multimerin-1 (MMRN1). Here, using AlphaFold2 screens, co-immunoprecipitation, enzymatic assays combined with mass spectrometric analysis and CRISPR-Cas9 knockouts, we demonstrate that FUT10 and FUT11, originally annotated in UniProt as α1,3-fucosyltransferases, are actually POFUTs responsible for modifying EMI domains; thus, we renamed them as POFUT3 and POFUT4, respectively. Like POFUT1/2, POFUT3/4 function in the endoplasmic reticulum, require folded domain structures for modification and participate in a non-canonical endoplasmic reticulum quality control pathway for EMI domain-containing protein secretion. This finding expands the O-fucosylation repertoire and provides an entry point for further exploration in this emerging field of O-fucosylation.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures






References
-
- Becker, D. J. & Lowe, J. B. Fucose: biosynthesis and biological function in mammals. Glycobiology13, 41R–53R (2003). - PubMed
-
- Li, J., Hsu, H. C., Mountz, J. D. & Allen, J. G. Unmasking fucosylation: from cell adhesion to immune system regulation and diseases. Cell Chem. Biol.25, 499–512 (2018). - PubMed
-
- Oriol, R. & Mollicone, R. Fucosyltransferases 1, 2. GDP-fucose galactoside α2-fucosyltransferases. FUT1 or H blood group, FUT2 or ABH secretor status and Sec1 (FUT1, FUT2, Sec1). In Handbook of Glycosyltransferases and Related Genes 2nd edn (eds Taniguchi, N. et al) 515–530 (Springer, 2014).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

