Knockdown of decorin in human bone marrow mesenchymal stem cells suppresses proteoglycan layer formation and establishes a pro-inflammatory environment on titanium oxide surfaces
- PMID: 39775189
- PMCID: PMC11706895
- DOI: 10.1007/s10856-024-06849-0
Knockdown of decorin in human bone marrow mesenchymal stem cells suppresses proteoglycan layer formation and establishes a pro-inflammatory environment on titanium oxide surfaces
Abstract
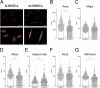
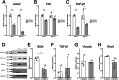
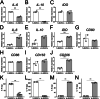
Osseointegration is essential for successful implant treatment. However, the underlying molecular mechanisms remain unclear. In this study, we focused on decorin (DCN), which was hypothesized to be present in the proteoglycan (PG) layer at the interface between bone and the titanium oxide (TiOx) surface. We utilized DCN RNA interference in human bone marrow mesenchymal stem cells (hBMSCs) to investigate its effects on PG layer formation, proliferation, initial adhesion, cell extension, osteogenic capacity, fibrotic markers, and immunotolerance to TiOx in vitro. After 14 days of cultivation, we observed no PG layer was detected, and the osteogenic capacity was suppressed in DCN-depleted hBMSCs. Furthermore, the conditioned medium upregulated the expression of M1 macrophage markers in human macrophages. These results suggest that endogenous DCN plays a crucial role in PG layer formation and that the PG layer alters inflammation around Ti materials.
© 2024. The Author(s).
Conflict of interest statement
Compliance with ethical standards. Conflict of interest: The authors declare no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

