Aggregating multiple test results to improve medical decision-making
- PMID: 39775197
- PMCID: PMC11741652
- DOI: 10.1371/journal.pcbi.1012749
Aggregating multiple test results to improve medical decision-making
Erratum in
-
Correction: Aggregating multiple test results to improve medical decision-making.PLoS Comput Biol. 2025 Aug 4;21(8):e1013347. doi: 10.1371/journal.pcbi.1013347. eCollection 2025 Aug. PLoS Comput Biol. 2025. PMID: 40758619 Free PMC article.
Abstract
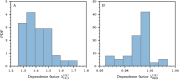
Gathering observational data for medical decision-making often involves uncertainties arising from both type I (false positive) and type II (false negative) errors. In this work, we develop a statistical model to study how medical decision-making can be improved by aggregating results from repeated diagnostic and screening tests. Our approach is relevant to not only clinical settings such as medical imaging, but also to public health, as highlighted by the need for rapid, cost-effective testing methods during the SARS-CoV-2 pandemic. Our model enables the development of testing protocols with an arbitrary number of tests, which can be customized to meet requirements for type I and type II errors. This allows us to adjust sensitivity and specificity according to application-specific needs. Additionally, we derive generalized Rogan-Gladen estimates of disease prevalence that account for an arbitrary number of tests with potentially different type I and type II errors. We also provide the corresponding uncertainty quantification.
Copyright: © 2025 Böttcher et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
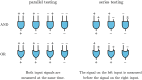
 ) and OR (
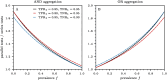
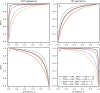
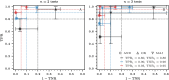
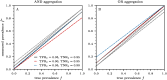
) and OR ( ). In parallel testing, both inputs are assessed simultaneously, while in series testing, the left input is examined before the right. Hence, if the initial test in a series protocol yields a negative result with aggregation through an AND gate, the assigned disease status will be negative, irrespective of the second input. In series testing with an OR gate, the assigned disease status will be positive if the first test is positive, regardless of the outcome of the second test.
). In parallel testing, both inputs are assessed simultaneously, while in series testing, the left input is examined before the right. Hence, if the initial test in a series protocol yields a negative result with aggregation through an AND gate, the assigned disease status will be negative, irrespective of the second input. In series testing with an OR gate, the assigned disease status will be positive if the first test is positive, regardless of the outcome of the second test.