Genome-based development and clinical evaluation of a customized LAMP panel to rapidly detect, quantify, and determine antibiotic sensitivity of Escherichia coli in native urine samples from urological patients
- PMID: 39775368
- PMCID: PMC11880174
- DOI: 10.1007/s10096-024-05030-3
Genome-based development and clinical evaluation of a customized LAMP panel to rapidly detect, quantify, and determine antibiotic sensitivity of Escherichia coli in native urine samples from urological patients
Abstract
Purpose: We designed and tested a point of care test panel to detect E.coli and antibiotic susceptibility in urine samples from patients at the point of care in the urological department. The aim of this approach is to facilitate choosing an appropriate antibiotic for urinary tract infections (UTI) at first presentation in the context of increasing antibiotic resistance in uropathogens worldwide.
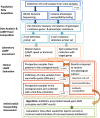
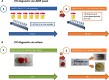
Methods: We analyzed 162 E.coli isolates from samples from a university urological department to determine phenotypic and genotypic resistance data. With this data we created customized LAMP (loop-mediated isothermal amplification) panels for a commercial machine with which to detect and possibly quantify E.coli and six antibiotic resistance determinants. In a second step we tested these panel(s) for diagnostic accuracy on 1596 urine samples and compared with routine microbiological culture.
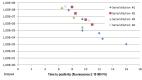
Results: E.coli was detected with 95.4% sensitivity and 96.1% specificity. Dynamics of the LAMP amplification could be used to gauge bacterial loads in the samples. Antibiotic sensitivity was detected with good negative (sensitive) predictive values: ampicillin 92.8%, ampicillin/sulbactam 96.4%, cefuroxime 92.8%, cefotaxime 97.8%, trimethoprim/sulfamethoxazole 96.5%, ciprofloxacin 96.8%.
Conclusion: The LAMP panel provided E.coli detection and sensitivity information within one hour and thus could principally guide initial antibiotic therapy upon patients presenting with UTI. The panel helps to select initial adequate antibiotic therapy as well as providing diagnostic stewardship. Follow up investigations will expand the test system to other uropathogens.
Keywords: Antibiotic susceptibility testing; Antimicrobial stewardship; Fast microbiology; Loop-mediated isothermal amplification; Point of care testing; Urinary tract infections.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: Our study has been approved by the ethics committee of the Faculty of Medicine, Justus-Liebig-University of Giessen, file number AZ 80/17. Competing interests: The authors declare no competing interests.
Figures


References
-
- Peterson J, Kaul S, Khashab M, Fisher A, Kahn JB (2007) Identification and Pretherapy Susceptibility of Pathogens in patients with complicated urinary tract infection or Acute Pyelonephritis enrolled in a clinical study in the United States from November 2004 through April 2006. Clin Ther 29(10):2215–2221. 10.1016/j.clinthera.2007.10.008 - DOI - PubMed
-
- Hooton TM, Bradley SF, Cardenas DD et al (2010) Diagnosis, prevention, and treatment of catheter-aassociated urinary tract infection in adults: 2009 international clinical practice guidelines from the infectious diseases society of America. Clin Infect Dis 50(5):625–663. 10.1086/650482 - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical

