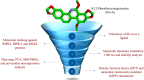
Targeting necroptosis in MCF-7 breast cancer cells: In Silico insights into 8,12-dimethoxysanguinarine from Eomecon Chionantha through molecular docking, dynamics, DFT, and MEP studies
- PMID: 39775383
- PMCID: PMC11706375
- DOI: 10.1371/journal.pone.0313094
Targeting necroptosis in MCF-7 breast cancer cells: In Silico insights into 8,12-dimethoxysanguinarine from Eomecon Chionantha through molecular docking, dynamics, DFT, and MEP studies
Abstract
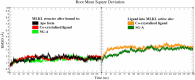
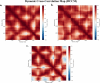
Breast cancer remains a significant challenge in oncology, highlighting the need for alternative therapeutic strategies that target necroptosis to overcome resistance to conventional therapies. Recent investigations into natural compounds have identified 8,12-dimethoxysanguinarine (SG-A) from Eomecon chionantha as a potential necroptosis inducer. This study presents the first computational exploration of SG-A interactions with key necroptotic proteins-RIPK1, RIPK3, and MLKL-through molecular docking, molecular dynamics (MD), density functional theory (DFT), and molecular electrostatic potential (MEP) analyses. Molecular docking revealed that SG-A exhibited a stronger affinity for MLKL (-9.40 kcal/mol) compared to the co-crystallized ligand (-6.29 kcal/mol), while its affinity for RIPK1 (-6.37 kcal/mol) and RIPK3 (-7.01 kcal/mol) was lower. MD simulations further demonstrated the stability of SG-A within the MLKL site, with RMSD values stabilizing between 1.4 and 3.3 Å over 300 ns, indicating a consistent interaction pattern. RMSF analysis indicated the preservation of protein backbone flexibility, with average fluctuations under 1.7 Å. The radius of gyration (Rg) results indicated a consistent value of ~15.3 Å across systems, confirming the role of SG-A in maintaining protein integrity. Notably, SG-A maintains two critical H-bonds within the active site of MLKL, reinforcing the stability of the interaction. Principal component analysis (PCA) indicated a significant reduction in MLKL's conformational space upon SG-A binding, implying enhanced stabilization. Dynamic cross-correlation map (DCCM) analysis further revealed that SG-A induced highly correlated motions, reducing internal fluctuations within MLKL compared to the co-crystallized ligand. MM-PBSA revealed the enhanced binding efficacy of SG-A, with a significant binding free energy of -31.03 ± 0.16 kcal/mol against MLKL, surpassing that of the control (23.96 ± 0.11 kcal/mol). In addition, the individual residue contribution analysis highlighted key interactions, with ARG149 showing a significant contribution (-176.24 kcal/mol) in the MLKL-SG-A complex. DFT and MEP studies corroborated these findings, revealing that the electronic structure of SG-A is conducive to stable binding interactions, characterized by a narrow band gap (~0.16 units) and distinct electrostatic potential favourable for necroptosis induction. In conclusion, SG-A has emerged as a compelling inducer of necroptosis for breast cancer therapy, warranting further experimental validation to fully realize its therapeutic potential.
Copyright: © 2025 Alhawarri et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Di Cosimo S. and Baselga J., Management of breast cancer with targeted agents: importance of heterogenicity. Nature reviews Clinical oncology, 2010. 7(3): p. 139–147. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous