Differentiating Between Perfluorohexyloctane Ophthalmic Solution and Water-Free Cyclosporine Ophthalmic Solution 0.1% for Dry Eye Disease: A Review of Preclinical and Clinical Characteristics
- PMID: 39775405
- PMCID: PMC11754776
- DOI: 10.1007/s40123-024-01076-w
Differentiating Between Perfluorohexyloctane Ophthalmic Solution and Water-Free Cyclosporine Ophthalmic Solution 0.1% for Dry Eye Disease: A Review of Preclinical and Clinical Characteristics
Abstract
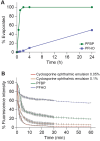
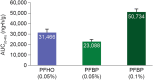
Perfluorohexyloctane ophthalmic solution (Miebo) and water-free cyclosporine ophthalmic solution 0.1% (Vevye) are recently approved treatments for dry eye disease (DED). Perfluorohexyloctane (PFHO) uses a novel approach to treat evaporative DED, whereas water-free cyclosporine (CsA 0.1%) is formulated to increase ocular delivery of its active ingredient to improve tear production. The two medications utilize the distinctive properties of two different semifluorinated alkanes (SFAs) to elicit their therapeutic effects. PFHO consists of 100% active ingredient and forms a monolayer on the surface of the tear film to inhibit evaporation. CsA 0.1% utilizes a vehicle consisting of perfluorobutylpentane (PFBP) and ethanol to facilitate delivery of cyclosporine to ocular tissues. The structure of these SFAs determines their differing behaviors and functions. The longer chain length of PFHO results in a slower evaporation rate and facilitates formation of a stable monolayer on the ocular surface. In vitro, PFHO demonstrated a substantially lower evaporation rate versus PFBP or human meibum, as well as a significantly longer ocular surface residence time. Ex vivo, PFHO demonstrated a longer ocular surface residence time than PFBP. The shorter chain length of PFBP enables it to better solubilize cyclosporine and improve drug delivery to ocular tissues. Although both of these ophthalmic drops utilize SFAs, their differences-in physicochemical properties and the mechanisms by which they are understood to intervene in the DED cycle-are important considerations in treatment selection for patients with DED.
Keywords: Anti-evaporative; Cyclosporine; Dry eye; Immunomodulator; Perfluorobutylpentane; Perfluorohexyloctane; Semifluorinated alkane.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: Laura M. Periman reports serving on advisory boards, speakers bureaus, and/or as a consultant for Alcon, Aldeyra Therapeutics, Allergan, Amgen, Azura Ophthalmics, Bausch + Lomb, Bruder Healthcare, Dompé, Eyedetec Medical, Kala Pharmaceuticals, Lumenis Be, Mallinckrodt, Myze, Novartis Pharmaceuticals, Nusight Medical, Olympic Ophthalmics, Science Based Health, Scope Health, Sun Pharmaceutical Industries, Tarsus, ThermaMEDx, Verséa, Visant, and Viatris; serving as a principal investigator for Alcon, Bausch + Lomb, Kala Pharmaceuticals, Lumenis Be, Novartis Pharmaceuticals, Olympic Ophthalmics, Tarsus, and Viatris; and is a shareholder of Eyedetec Medical, Myze, Quench Method, ThermaMEDx, Verséa, and Visant. Darrell E. White reports serving as a consultant and speaker to Bausch + Lomb. Douglas Katsev reports serving as a consultant and speaker to Bausch + Lomb. Douglas Katsev has no financial interest in the subject matter of this manuscript. The authors report no other conflicts of interest in this work. Ethical Approval: This article is based on previously conducted studies and does not contain any new studies performed by any of the authors on human participants or animals.
Figures
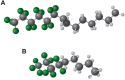
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

