SARS-CoV-2-induced cytokine storm drives prolonged testicular injury and functional impairment in mice that are mitigated by dexamethasone
- PMID: 39775442
- PMCID: PMC11706467
- DOI: 10.1371/journal.ppat.1012804
SARS-CoV-2-induced cytokine storm drives prolonged testicular injury and functional impairment in mice that are mitigated by dexamethasone
Abstract
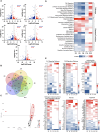
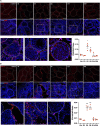
Compromised male reproductive health, including reduced testosterone and sperm count, is one of the long COVID symptoms in individuals recovering from mild-severe disease. COVID-19 patients display testicular injury in the acute stage and altered serum fertility markers in the recovery phase, however, long-term implications on the testis remain unknown. This study characterized the consequences of SARS-CoV-2 on testis function. The K18-hACE2 mice that survived SARS-CoV-2 infection were followed for one month after infection and the testicular injury and function markers were assessed at different stages of infection and recovery. The long-term impact of infection on key testes function-related hormones and male fertility was measured. The efficacy of inflammation-suppressing drug in preventing testicular injury was also evaluated. The morphological defects like sloughing of spermatids into the lumen and increased apoptotic cells sustained for 2-4 weeks after infection and correlated with testicular inflammation and immune cell infiltration. Transcriptomic analysis revealed dysregulation of inflammatory, cell death, and steroidogenic pathways. Furthermore, reduced testosterone levels associated with a transient reduction in sperm count and male fertility. Most testicular impairments resolved within one month of infection. Importantly, dexamethasone treatment attenuated testicular damage, inflammation, and immune infiltration. Our results implicate virus-induced cytokine storm as the major driver of testicular injury and functional impairments, timely prevention of which limits testis damage. These findings serve as a model for evaluating therapeutics in long COVID patients and may guide clinical strategies to improve male reproductive health outcomes post-SARS-CoV-2 infection.
Copyright: © 2025 Giannakopoulos et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

