Advances in human papillomavirus detection for cervical cancer screening and diagnosis: challenges of conventional methods and opportunities for emergent tools
- PMID: 39775553
- PMCID: PMC11706323
- DOI: 10.1039/d4ay01921k
Advances in human papillomavirus detection for cervical cancer screening and diagnosis: challenges of conventional methods and opportunities for emergent tools
Abstract
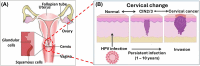
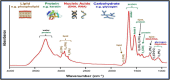
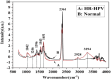
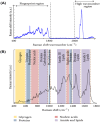
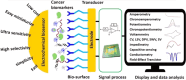
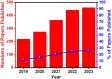
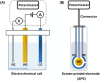
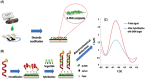
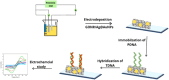
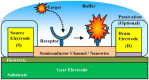
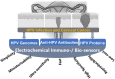
Human papillomavirus (HPV) infection is the main cause of cervical cancer and other cancers such as anogenital and oropharyngeal cancers. The prevention screening and treatment of cervical cancer has remained one of the top priorities of the World Health Organization (WHO). In 2020, the WHO came up with the 90-70-90 strategy aimed at eliminating cervical cancers as a public health problem by the year 2030. One of the key priorities of this strategy is the recommendation for countries to ensure that 70% of their women are screened using a high-performance test by the age of 35, and again by the age of 45. Over the years, several traditional methods (notably, Pap smear and nucleic acid-based techniques) have been used for the detection of cervical cancer. While these methods have significantly reduced the incidence of cervical cancer and death, they still come short of excellence for the total eradication of HPV infection. The challenges include low sensitivity, low specificity, poor reproducibility, the need for high-level specialists, and the high cost of access to the facilities, to mention a few. Interestingly, however, several efforts are being made today to mitigate these challenges. In this review, we discussed the pros and cons of the traditional screening and testing of HPV infections, the efforts being made to improve their performances, and the emergent tools (especially, the electrochemical methods) that promise to revolutionize the screening and testing of HPV infections. The main aim of the review is to provide some novel clues to researchers that would allow for the development of high-performance, affordable, and triage-suitable electrochemical-based diagnostic tools for HPV and cervical cancer.
Conflict of interest statement
There are no conflicts to declare.
Figures




Similar articles
-
Use of a high-risk human papillomavirus DNA test as the primary test in a cervical cancer screening programme: a population-based cohort study.BJOG. 2013 Sep;120(10):1260-7; discussion 1267-8. doi: 10.1111/1471-0528.12272. Epub 2013 Jun 21. BJOG. 2013. PMID: 23786222
-
Clinical evaluation of primary human papillomavirus (HPV) testing with extended HPV genotyping triage for cervical cancer screening: A pooled analysis of individual patient data from nine population-based cervical cancer screening studies from China.Cancer Med. 2024 Jun;13(11):e7316. doi: 10.1002/cam4.7316. Cancer Med. 2024. PMID: 38828559 Free PMC article.
-
Overview of human papillomavirus-based and other novel options for cervical cancer screening in developed and developing countries.Vaccine. 2008 Aug 19;26 Suppl 10:K29-41. doi: 10.1016/j.vaccine.2008.06.019. Vaccine. 2008. PMID: 18847555 Review.
-
Human papillomavirus testing for primary cervical cancer screening.Expert Rev Mol Diagn. 2008 Sep;8(5):599-605. doi: 10.1586/14737159.8.5.599. Expert Rev Mol Diagn. 2008. PMID: 18785808 Review.
-
Comparison of human papillomavirus genotyping and cytology triage, COMPACT Study: Design, methods and baseline results in 14 642 women.Cancer Sci. 2018 Jun;109(6):2003-2012. doi: 10.1111/cas.13608. Epub 2018 May 31. Cancer Sci. 2018. PMID: 29660849 Free PMC article.
Cited by
-
HPV-Associated Sexually Transmitted Infections in Cervical Cancer Screening: A Prospective Cohort Study.Viruses. 2025 Feb 11;17(2):247. doi: 10.3390/v17020247. Viruses. 2025. PMID: 40007002 Free PMC article.
-
Trends in HPV-positive cervical cancer prevalence: a retrospective study from 2013 to 2020.Virol J. 2025 Jun 18;22(1):199. doi: 10.1186/s12985-025-02830-7. Virol J. 2025. PMID: 40533808 Free PMC article.
-
Medicinal Mushrooms, Probiotics and Combination of Natural Compounds in the Management of HPV: A Comparative Look at Viral Clearance and Lesion Resolution.Viruses. 2025 Jul 2;17(7):942. doi: 10.3390/v17070942. Viruses. 2025. PMID: 40733559 Free PMC article. Review.
-
Evaluating TGF-β1 gene expression and promoter polymorphism in cervical cancer progression.J Mol Histol. 2025 Apr 2;56(2):126. doi: 10.1007/s10735-025-10402-w. J Mol Histol. 2025. PMID: 40172705
References
-
- Arumugam M. R. K. Suvankar Ghora G. Saikat Kumar J. J. Electrochem. Soc. 2024;171:027519. doi: 10.1149/1945-7111/ad281b. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

