Mesothelial cell responses to acute appendicitis or small bowel obstruction reactive ascites: Insights into immunoregulation of abdominal adhesion
- PMID: 39775680
- PMCID: PMC11709316
- DOI: 10.1371/journal.pone.0317056
Mesothelial cell responses to acute appendicitis or small bowel obstruction reactive ascites: Insights into immunoregulation of abdominal adhesion
Abstract
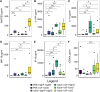
Previous abdominal surgery (PAS) increases risk of small bowel obstruction (SBO) due to adhesions, and appendectomy (appy) is an independent risk factor for abdominal adhesion-related complications. Peritoneal inflammation, e.g., acute appendicitis (AA), causes formation of reactive ascitic fluid (rA) that activates peritoneum surface mesothelial cells (MCs) to form adhesions. Pathologic adhesions may arise if restoration of MC-regulated fibrinolysis and secretion of glycocalyx (GCX) are disrupted. Proteins affecting these processes may originate from peritoneal rA. This is a prospective observational IRB-approved study at three Level 1 trauma centers where rA is collected prior to surgical intervention for non-perforated AA or adhesiolysis for SBO. Samples from 48 appy and 15 SBO patients were used to treat human MCs and subjected to quantification of 85 inflammatory mediators. Results were compared between patients with surgically naïve abdomens (naïve) and patients with >1 PAS. Select rA caused MCs to form clusters of fibroblastic cells, extracellular matrix fibers (FIB), and secretion of GCX. PAS and naïve patient rA fluids were clustered into "fiber-GCX" (FIB-GCX) groups: highFIB-highGCX, highFIB-lowGCX, noFIB-highGCX, noFIB-lowGCX, and noFIB-noGCX. Between groups, 26 analytes were differentially abundant including innate immune response, wound healing, and mucosal defense proteins. Factors that contributed to the differences between groups were rA-induced highFIB and history of PAS. Overall, PAS patient rA showed a muted immune response compared to rA from naïve patients. Our data suggest that abdominal surgery may negatively impact future immune responses in the abdomen. Further, quantifying immunomodulators in peritoneal rA may lead to the development a personalized approach to post-surgical adhesion treatment and prevention.
Copyright: © 2025 Hausburg et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Ellis H. The clinical significance of adhesions: focus on intestinal obstruction. Eur J Surg Suppl. 1997;(577):5–9. Epub 1997/01/01. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

