Chronic Jet Lag Disrupts Circadian Rhythms and Induces Hyperproliferation in Murine Lacrimal Glands via ROS Accumulation
- PMID: 39775698
- PMCID: PMC11717126
- DOI: 10.1167/iovs.66.1.12
Chronic Jet Lag Disrupts Circadian Rhythms and Induces Hyperproliferation in Murine Lacrimal Glands via ROS Accumulation
Abstract
Purpose: Chronic jet lag (CJL) is known to disrupt circadian rhythms, which regulate various physiological processes, including ocular surface homeostasis. However, the specific effects of CJL on lacrimal gland function and the underlying cellular mechanisms remain poorly understood.
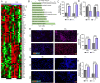
Methods: A CJL model was established using C57BL/6J mice. Extraorbital lacrimal glands (ELGs) were collected at 3-hour intervals for RNA extraction and high-throughput RNA sequencing. Circadian transcriptomic profiles were analyzed, and functional annotations were performed. Hydrogen peroxide levels and total antioxidant capacity in tear fluid were measured using chemometric assays. Immunofluorescence was used to assess cell proliferation, apoptosis, immune cell infiltration in ELGs, and reactive oxygen species (ROS) accumulation. The potential therapeutic effects of alpha-lipoic acid (ALA) on CJL-induced oxidative stress and pathological changes in ELGs were also investigated.
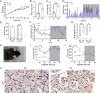
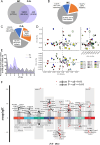
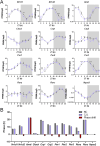
Results: CJL significantly disrupted locomotor activity, altered body temperature rhythms, and modified diurnal oscillations in ELGs. Transcriptomic analysis revealed extensive changes in rhythmic gene expression, phase shifts, and pathway clustering in response to CJL. The disruption of the core circadian clock transcription was associated with ELG hyperproliferation and increased ROS accumulation. tert-Butyl hydroperoxide promoted ELG cell proliferation, and ALA effectively reduced ROS levels and mitigated CJL-induced hyperproliferation.
Conclusions: These findings uncover novel molecular pathways affected by CJL and highlight the potential of antioxidant therapies, such as ALA, in preserving ocular surface health under conditions of circadian rhythm disruption.
Conflict of interest statement
Disclosure:
Figures








References
-
- Patke A, Young MW, Axelrod S.. Molecular mechanisms and physiological importance of circadian rhythms. Nat Rev Mol Cell Biol . 2020; 21(2): 67–84. - PubMed
-
- Sack RL. Clinical practice. Jet lag. N Engl J Med . 2010; 362(5): 440–447. - PubMed
-
- Waterhouse J, Reilly T, Atkinson G, Edwards B.. Jet lag: trends and coping strategies. Lancet . 2007; 369(9567): 1117–1129. - PubMed
-
- Filipski E, Delaunay F, King VM, et al.. Effects of chronic jet lag on tumor progression in mice. Cancer Res . 2004; 64(21): 7879–7885. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

