Effects of an Initial Anti-CD19 CAR T-cell Therapy on Subsequent Anti-CD22 CAR T-cell Manufacturing and Clinical Outcomes in Patients with Relapsed/Refractory LBCL
- PMID: 39775812
- PMCID: PMC11964843
- DOI: 10.1158/2159-8290.CD-24-1071
Effects of an Initial Anti-CD19 CAR T-cell Therapy on Subsequent Anti-CD22 CAR T-cell Manufacturing and Clinical Outcomes in Patients with Relapsed/Refractory LBCL
Abstract
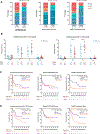
Late leukapheresis (>6 months after CAR19) resulted in less residual CAR19, higher CAR22 CD4+ naïve T and TCM cells, less TEM cells, and higher CD8+ TCM cells, but similar clinical outcomes to those with early leukapheresis. CAR22 responses were associated with higher transduction efficiency and CD8+ TCM and less CD8+ TEM cells.
©2025 American Association for Cancer Research.
Conflict of interest statement
Disclosure of Conflicts of Interest
A.M.K.: Patent rights for CAT CAR in targeting CD19 and receive royalties from Autolus Therapeutics PLC.
M.P.H.: Consulting or Advisory Role: Kite Pharmaceuticals.
J.H.B.: Consulting for Kite Pharma-Gilead. Research support from Kite Pharma-Gilead, Cargo Therapeutics, Genentech-Roche, Regeneron Pharmaceuticals, and Janssen.
L.M.: Research support from Adaptive Biotechnologies and Servier Laboratories; consulting for Amgen, Pfizer.
C.L.M.: Founder, equity, consulting for Cargo Therapeutics. Founder, equity, consulting and Director of Link Cell Therapies. Royalties from NIH, for CAR22. Consulting for Immatics, Ensoma. Research funding from Lyell Immunopharma and Tune Therapeutics.
Z.G.: Consulting for Mubadala Ventures and Boom Capital Ventures. Research support from Kite Pharma-Gilead and 10x Genomics. Honoraria from Standard Biotools and Sangamo Therapeutics.
S.A.F.: Consulting or Advisory Role: Autolomous, FreshWind Bio, MicroFluidX (MFX), Achieve Clinics, University of Oslo (Center for Advanced Cell Therapy), Genentech and Lyell.
D.B.M.: Consulting for Kite Pharma-Gilead, Juno Therapeutics-Celgene, Novartis, Janssen, and Pharmacyclics. Research support from Kite Pharma-Gilead, Allogene, Cargo therapeutics, Pharmacyclics, Miltenyi Biotec, and Adaptive Biotechnologies.
M.J.F.: Consulting for Kite Pharma-Gilead, Adaptative Biotechnologies, ADC Therapeutics and Cargo Therapeutics. Research support from Kite-Pharma-Gilead, Allogene Therapeutics, CARGO Therapeutics and Adaptative Biotechnologies.
Y.J.S, N.A., H.K.S., B.S., A.K., Z.J.E., M.H.D., S.P.R., R.T., S.P., H.C., H.G.L., D.D.K., A.K.B., S.B., S.D., and M.S. have no competing financial interests to declare.
Figures
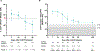



References
-
- Westin JR, Oluwole OO, Kersten MJ, Miklos DB, Perales M-A, Ghobadi A, et al. Survival with Axicabtagene Ciloleucel in Large B-Cell Lymphoma. N Engl J Med 2023;389(2):148–57 - PubMed
-
- Schuster SJ, Tam CS, Borchmann P, Worel N, McGuirk JP, Holte H, et al. Long-term clinical outcomes of tisagenlecleucel in patients with relapsed or refractory aggressive B-cell lymphomas (JULIET): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol 2021;22(10):1403–15 - PubMed
-
- Abramson JS, Palomba ML, Gordon LI, Lunning M, Wang M, Arnason J, et al. Two-year follow-up of lisocabtagene maraleucel in relapsed or refractory large B-cell lymphoma in TRANSCEND NHL 001. Blood 2024;143(5):404–16 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

