Clinical 7 Tesla magnetic resonance imaging: Impact and patient value in neurological disorders
- PMID: 39775908
- PMCID: PMC11846079
- DOI: 10.1111/joim.20059
Clinical 7 Tesla magnetic resonance imaging: Impact and patient value in neurological disorders
Abstract
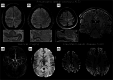
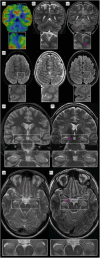
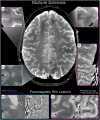
Magnetic resonance imaging (MRI) is a cornerstone of non-invasive diagnostics and treatment monitoring, particularly for diseases of the central nervous system. Although 1.5- and 3 Tesla (T) field strengths remain the clinical standard, the advent of 7 T MRI represents a transformative step forward, offering superior spatial resolution, contrast, and sensitivity for visualizing neuroanatomy, metabolism, and function. Recent innovations, including parallel transmission and deep learning-based reconstruction, have resolved many prior technical challenges of 7 T MRI, enabling its routine clinical use. This review examines the diagnostic impact, patient value, and practical considerations of 7 T MRI, emphasizing its role in facilitating earlier diagnoses and improving care in conditions, such as amyotrophic lateral sclerosis (ALS), epilepsy, multiple sclerosis (MS), dementia, parkinsonism, tumors, and vascular diseases. Based on insights from over 1200 clinical scans with a second-generation 7 T system, the review highlights disease-specific biomarkers such as the motor band sign in ALS and the new diagnostic markers in MS, the central vein sign, and paramagnetic rim lesions. The unparalleled ability of 7 T MRI to study neurological diseases ex vivo at ultra-high resolution is also explored, offering new opportunities to understand pathophysiology and identify novel treatment targets. Additionally, the review provides a clinical perspective on patient handling and safety considerations, addressing challenges and practicalities associated with clinical 7 T MRI. By bridging research and clinical practice, 7 T MRI has the potential to redefine neuroimaging and advance the understanding and management of complex neurological disorders.
Keywords: dementia; diagnosis; multiple sclerosis; neurology; radiology; vascular disease.
© 2025 The Author(s). Journal of Internal Medicine published by John Wiley & Sons Ltd on behalf of Association for Publication of The Journal of Internal Medicine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Assessment of the available evidence for the use of 7-Tesla (T) magnetic resonance imaging (MRI) in neurological and musculoskeletal disorders, with comparison to 3-T and 1.5-T MRI: A systematic scoping review.Eur J Neurol. 2025 Jan;32(1):e16557. doi: 10.1111/ene.16557. Eur J Neurol. 2025. PMID: 39676509 Free PMC article.
-
Magnetic Resonance Iron Imaging in Amyotrophic Lateral Sclerosis.J Magn Reson Imaging. 2022 May;55(5):1283-1300. doi: 10.1002/jmri.27530. Epub 2021 Feb 15. J Magn Reson Imaging. 2022. PMID: 33586315 Review.
-
Advanced neuroimaging approaches in amyotrophic lateral sclerosis: refining the clinical diagnosis.Expert Rev Neurother. 2020 Mar;20(3):237-249. doi: 10.1080/14737175.2020.1715798. Epub 2020 Jan 17. Expert Rev Neurother. 2020. PMID: 31937156 Review.
-
Clinical applications of ultra-high field magnetic resonance imaging in multiple sclerosis.Expert Rev Neurother. 2018 Mar;18(3):221-230. doi: 10.1080/14737175.2018.1433033. Epub 2018 Jan 30. Expert Rev Neurother. 2018. PMID: 29369733 Free PMC article. Review.
-
Magnetic Resonance Imaging of Multiple Sclerosis at 7.0 Tesla.J Vis Exp. 2021 Feb 19;(168). doi: 10.3791/62142. J Vis Exp. 2021. PMID: 33682856
References
-
- Williams SN, Allwood‐Spiers S, Mcelhinney P, Paterson G, Herrler J, Liebig P, Nagel AM, et al. A nested eight‐channel transmit array with open‐face concept for human brain imaging at 7 Tesla. Front Phys. 2021;9:701330.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

