The efficacy of lavender oil on fatigue and sleep quality in patients with hematological malignancy receiving chemotherapy: a single-blind randomized controlled trial
- PMID: 39775962
- PMCID: PMC11711766
- DOI: 10.1007/s00520-024-09143-5
The efficacy of lavender oil on fatigue and sleep quality in patients with hematological malignancy receiving chemotherapy: a single-blind randomized controlled trial
Abstract
Purpose: The aim of this study is to evaluate how aromatherapy with the inhalation of lavender oil affects fatigue and sleep quality in patients with hematological malignancies undergoing chemotherapy.
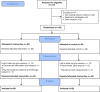
Methods: This randomized, parallel-group study was carried out in the Adult Bone Marrow Transplant unit and Hematology-Oncology clinics between January 2022 and April 2023. A total of 120 patients were assigned to experimental and control groups by randomization. The study was completed with 100 patients including 50 in the experimental group and 50 in the control group. Lavender essential oil was applied to the experimental group for 20 min prior to going to bed every night for 5 consecutive days. Physiological saline solution was applied to the control group in the same way. A Participant Information Form, the Richards-Campbell Sleep Questionnaire, and the Piper Fatigue Scale were used as data collection tools.
Results: The experimental group showed a significantly higher sleep quality (p = 0.001) and had a significantly lower PFS scores (p = 0.001) compared to the control group. Also, the mean scores of the experimental group on the Behavioral, Sensory, and Cognitive subscales were statistically significantly lower than the scores of the control group (p < 0.05). Variables of lavender aromatherapy and total sleep quality accounted for 17.1% of the variance in fatigue levels (R2 = 0.171).
Conclusions: Consequently, it was determined that aromatherapy with lavender essential oil significantly alleviated fatigue and lowered PFS total and subscale scores in patients with hematological malignancies undergoing chemotherapy. Also, sleep quality significantly enhanced in the overall PFS and its subscales.
Trial registration: NCT05808296. Date of Registration: 30 March 2023.
Keywords: Aromatherapy; Chemotherapy; Fatigue; Hematological malignancies; Lavender oil; Sleep disorders; Sleep quality.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval: Approval was obtained from Sinop University Ethics Committee (Number 2021/72). All procedures in this study were performed in accordance with the ethical standards of the institutional research committee and with the Declaration of Helsinki and its later amendments or similar ethical standards. Consent to participate: Informed consent was obtained from all participants included in the study. Competing interest: The authors declare no competing interests.
References
-
- Sung H, Ferlay J, Siegel RL et al (2021) Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249. 10.3322/caac.21660 - PubMed
-
- Noyan S, Gündogdu F, Bozdağ SC (2023) The level of fatigue, insomnia, depression, anxiety, stress, and the relationship between these symptoms following allogeneic hematopoietic stem cell transplantation: a cross-sectional study. Support Care Cancer 31:244. 10.1007/S00520-023-07703-9 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous