Patient and Public Involvement in Pharmacoepidemiological Research: An Environmental Scan of an Emerging Area
- PMID: 39776060
- PMCID: PMC11706696
- DOI: 10.1002/pds.70080
Patient and Public Involvement in Pharmacoepidemiological Research: An Environmental Scan of an Emerging Area
Abstract
Background: Patient and public involvement (PPI) in research is required to improve the relevance, feasibility, and interpretability. However, research on PPIs in pharmacoepidemiology (PE) is still limited. This study aimed to provide an overview of PPI implementation in pharmacoepidemiology through an environmental scan.
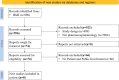
Methods: The environmental scan combined systematic reviews and expert interviews. A systematic review was conducted in MEDLINE, EMBASE, and the Cochrane Library from January 1, 2010, to June 30, 2023, to identify PE studies in which PPIs were mentioned. An additional review covered British Medical Journal's (BMJ) original articles from January 1, 2019, to June 30, 2023, via a similar method. In parallel, a cross-sectional study was conducted with a standardized questionnaire for French PE research teams, involving interviews via videoconference.
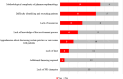
Results: We identified 3615 references for screening, among which 232 were selected for full-text screening. However, no studies have reported the use of PPIs in PE studies. The additional BMJ review identified 1058 references, 74 of which met the full-text selection criteria, and eight were included. Of 13 French PE research teams surveyed, three had prior PPI experience, and 12 affirmed the relevance of PPI in PE. The respondents identified barriers such as PE's complexity (n = 9). They suggested training for patients (n = 9) and collaboration with specialist teams (n = 5) to facilitate PPI.
Conclusion: Our environmental scan highlighted the emergence and relevance of PPIs in PE studies, even though they are still uncommon. Tools are needed to acculture and assist PE researchers in engaging in PPIs.
Keywords: methods; patient and public involvment; pharmacoepidemiology.
© 2025 The Author(s). Pharmacoepidemiology and Drug Safety published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest. This manuscript represents the views of the authors and does not necessarily represent the opinion of the French Medicines Agency.
Figures
References
-
- NIHR , “Briefing Notes for Researchers,” (2021).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous