SARS-CoV-2 Activated Peripheral Blood Mononuclear Cells (PBMCs) Do Not Provoke Adverse Effects in Trophoblast Spheroids
- PMID: 39776066
- PMCID: PMC11706221
- DOI: 10.1111/aji.70039
SARS-CoV-2 Activated Peripheral Blood Mononuclear Cells (PBMCs) Do Not Provoke Adverse Effects in Trophoblast Spheroids
Abstract
Problem: Although it is still uncertain whether Severe Acute Respiratory Coronavirus (SARS-CoV-2) placental infection and vertical transmission occur, inflammation during early pregnancy can have devastating consequences for gestation itself and the growing fetus. If and how SARS-CoV-2-specific immune cells negatively affect placenta functionality is still unknown.
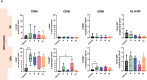
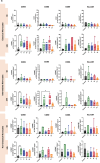
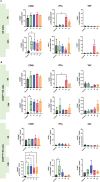
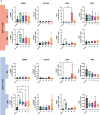
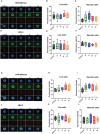
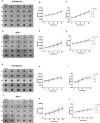
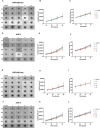
Method of study: We stimulated peripheral blood mononuclear cells (PBMCs) from women of reproductive age with SARS-CoV-2 peptides and cocultured them with trophoblast spheroids (HTR-8/SVneo and JEG-3) to dissect if SARS-CoV-2-activated immune cells can interfere with trophoblast functionality. The activation and cytokine profile of the PBMCs were determined using multicolor flow cytometry. The functionality of trophoblast spheroids was assessed using microscopy, enzyme-linked immunosorbent assay (ELISA), and RT-qPCR.
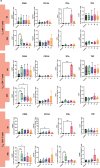
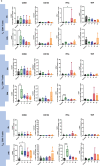
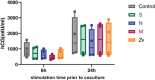
Results: SARS-CoV-2 S and M peptides significantly activated PBMCs (monocytes, NK cells, and T cells with memory subsets) and induced the upregulation of proinflammatory cytokines, such as IFNγ. The activated PBMCs did not impact the viability, growth rate, and invasion capabilities of trophoblast spheroids. Furthermore, the hormonal production of hCG by JEG-3 spheroids was not compromised upon coculture with the activated PBMCs. mRNA transcript levels of genes involved in trophoblast spheroid functional pathways were also not dysregulated after coculture.
Conclusions: Together, the findings of our in vitro coculture model, although not fully representative of in vivo conditions, strongly support the claim that the interaction of SARS-CoV-2-activated peripheral blood immune cells with trophoblast cells at the fetal-maternal interface does not negatively affect trophoblast functionality. This goes in hand with the recommendation of vaccinating pregnant women in their first trimester.
Keywords: 3D cell culture; SARS‐CoV‐2; peripheral blood mononuclear cells (PBMCs); pregnancy; spheroids; trophoblast invasion; trophoblast viability.
© 2025 The Author(s). American Journal of Reproductive Immunology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- WHO , COVID‐19 Cases | WHO COVID‐19 Dashboard (2024), https://data.who.int/dashboards/covid19/cases?n=c.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

