Emergency department-initiated oral naltrexone for patients with moderate to severe alcohol use disorder: A pilot feasibility study
- PMID: 39776077
- PMCID: PMC12077059
- DOI: 10.1111/acem.15059
Emergency department-initiated oral naltrexone for patients with moderate to severe alcohol use disorder: A pilot feasibility study
Abstract
Objectives: Alcohol use disorder (AUD) is the most common substance use disorder in the United States. Despite availability of four FDA-approved medications, fewer than 10% of patients are prescribed medication. This study aimed to evaluate the impact and feasibility of emergency department (ED)-initiated oral naltrexone in patients with moderate to severe AUD.
Methods: This was a prospective, single-arm, open-label, nonrandomized clinical trial conducted a single ED. Consenting participants were adults with moderate to severe AUD who were provided a single 50-mg dose of oral naltrexone, a 14-day starter pack of naltrexone, and referral for treatment. Follow-up was conducted at 14 and 30 days post-ED visit. The primary outcome was engagement in formal addiction treatment. Secondary outcomes included alcohol consumption, craving, quality-of-life measures, satisfaction, and safety.
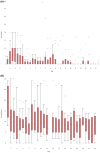
Results: Of 761 patients screened, 21 enrolled and received at least one dose of naltrexone. At 14 days, 29% were engaged in treatment, increasing to 33% at 30 days. There was a decrease in the mean (±SD) number of drinks per day from 5.20 (±4.67) at baseline to 2.23 (±4.35) during the follow-up period (p = 0.078). There was a decrease in alcohol craving scores, with median scores dropping from 19 at baseline to 8.27 during the follow-up period (p < 0.001). Quality-of-life measures improved, with a statistically significant increase in the reported number of healthy days (p = 0.006) and decrease in depressive symptoms (p < 0.001). Reported side effects were mild and satisfaction with the screening process was high.
Conclusions: ED-initiated oral naltrexone is feasible and acceptable for patients with moderate to severe AUD. While engagement in treatment was moderate, significant reductions in alcohol craving and improvements in quality of life suggest potential benefits. Further research is warranted to confirm these findings.
Keywords: alcohol use disorder; emergency service; hospital; medication‐assisted treatment; naltrexone.
© 2025 The Author(s). Academic Emergency Medicine published by Wiley Periodicals LLC on behalf of Society for Academic Emergency Medicine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Poznyak V, Rekve D. Global Status Report on Alcohol and Health 2018. World Health Organization; 2018.
-
- Substance Abuse and Mental Health Services Administration . Key Substance Use and Mental Health Indicators in the United States: Results from the 2021 National Survey on Drug Use and Health. Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration; 2021.
-
- Sacks JJ, Gonzales KR, Bouchery EE, Tomedi LE, Brewer RD. 2010 national and state costs of excessive alcohol consumption. Am J Prev Med. 2015;49:e73‐e79. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

