Magnetotactic bacteria affiliated with diverse Pseudomonadota families biomineralize intracellular Ca-carbonate
- PMID: 39776138
- PMCID: PMC11773610
- DOI: 10.1093/ismejo/wrae260
Magnetotactic bacteria affiliated with diverse Pseudomonadota families biomineralize intracellular Ca-carbonate
Abstract
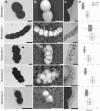
Intracellular calcium carbonate formation has long been associated with a single genus of giant Gammaproteobacteria, Achromatium. However, this biomineralization has recently received increasing attention after being observed in photosynthetic Cyanobacteriota and in two families of magnetotactic bacteria affiliated with the Alphaproteobacteria. In the latter group, bacteria form not only intracellular amorphous calcium carbonates into large inclusions that are refringent under the light microscope, but also intracellular ferrimagnetic crystals into organelles called magnetosomes. Here new observations suggest that magnetotactic bacteria previously identified in the sediments and water column of Lake Pavin (France) were only a small fraction of the diversity of bacteria producing intracellular amorphous calcium carbonates. To explore this diversity further, we conducted a comprehensive investigation of magnetotactic populations with refractive granules using a combination of environmental microbiology, genomic and mineralogy approaches on cells sorted by micromanipulation. Several species belonging to divergent genera of two Pseudomonadota classes were identified and characterized. Scanning transmission electron microscopy coupled with energy-dispersive X-ray spectrometry support that all these species indeed form intracellular amorphous calcium carbonates. Cryo soft X-ray tomography experiments conducted on ice-vitrified cells, enabled 3D investigation of inclusions volume, which was found to occupy 44-68% of the cell volume. Metabolic network modeling highlighted different metabolic abilities of Alpha- and Gammaproteobacteria, including methylotrophy and CO2 fixation via the reverse Krebs cycle or the Calvin-Benson-Bassham cycle. Overall, this study strengthens a convergent evolution scenario for intracellular carbonatogenesis in Bacteria, and further supports that it is promoted by the fixation of CO2 in anoxic environments.
Keywords: biomineralization; calcium carbonate; carbonatogenesis; environmental microbiology; magnetotactic bacteria.
© The Author(s) 2025. Published by Oxford University Press on behalf of the International Society for Microbial Ecology.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures





References
-
- Berenjian A, Seifan M. Mineral Formation by Microorganisms: Concepts and Applications. Switzerland: Springer Nature, 2022.
-
- Görgen S, Benzerara K, Skouri-Panet F et al. The diversity of molecular mechanisms of carbonate biomineralization by bacteria. Discov Mater 2020;1:2. 10.1007/s43939-020-00001-9 - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

