Updated appropriate use criteria for amyloid and tau PET: A report from the Alzheimer's Association and Society for Nuclear Medicine and Molecular Imaging Workgroup
- PMID: 39776249
- PMCID: PMC11772739
- DOI: 10.1002/alz.14338
Updated appropriate use criteria for amyloid and tau PET: A report from the Alzheimer's Association and Society for Nuclear Medicine and Molecular Imaging Workgroup
Abstract
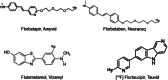
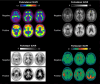
Introduction: The Alzheimer's Association and the Society of Nuclear Medicine and Molecular Imaging convened a multidisciplinary workgroup to update appropriate use criteria (AUC) for amyloid positron emission tomography (PET) and to develop AUC for tau PET.
Methods: The workgroup identified key research questions that guided a systematic literature review on clinical amyloid/tau PET. Building on this review, the workgroup developed 17 clinical scenarios in which amyloid or tau PET may be considered. A modified Delphi approach was used to rate each scenario by consensus as "rarely appropriate," "uncertain," or "appropriate." Ratings were performed separately for amyloid and tau PET as stand-alone modalities.
Results: For amyloid PET, seven scenarios were rated as appropriate, two as uncertain, and eight as rarely appropriate. For tau PET, five scenarios were rated as appropriate, six as uncertain, and six as rarely appropriate.
Discussion: AUC for amyloid and tau PET provide expert recommendations for clinical use of these technologies in the evolving landscape of diagnostics and therapeutics for Alzheimer's disease.
Highlights: A multidisciplinary workgroup convened by the Alzheimer's Association and the Society of Nuclear Medicine and Molecular Imaging updated the appropriate use criteria (AUC) for amyloid positron emission tomography (PET) and to develop AUC for tau PET. The goal of these updated AUC is to assist clinicians in identifying clinical scenarios in which amyloid or tau PET may be useful for guiding the diagnosis and management of patients who have, or are at risk for, cognitive decline These updated AUC are intended for dementia specialists who spend a significant proportion of their clinical effort caring for patients with cognitive complaints, as well as serve as a general reference for a broader audience interested in implementation of amyloid and tau PET in clinical practice.
Keywords: Alzheimer's disease; PET imaging; amyloid PET; appropriate use criteria; biomarkers; brain pathology; clinical care; cognitive impairment; dementia; diagnosis; early detection; memory disorders; molecular imaging; neuroimaging; neurology; positron emission tomography; tau PET; therapeutic strategies; treatment.
© 2025 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
The workgroup members were required to provide disclosure statements of all relationships that might be perceived as a real or potential COI. These statements were reviewed and discussed by the workgroup co‐chairs and updated and reviewed by an objective third party at the beginning of every task force meeting and/or teleconference. Disclosures for task force members can be found in the table below.
To adjudicate the COIs, the leadership from the AA, SNMMI, and Avalere first determined the threshold for a real COI. Following consultation with various experts and review of other policies used, the team defined COIs as the following: An individual that had relationships with industry, including consulting, speaking, research, and other non‐research activities, that exceed $5,000 in funding over the previous or upcoming 12‐month period. [Table: see text]
The AA, SNMMI, and Avalere rigorously attempted to avoid any actual, perceived, or potential conflicts of interest (COIs) that might have arisen because of an outside relationship or personal interest of workgroup members. Both organizations reviewed their own industry relationship policies to ensure that the ensuing process adhered to both standards. Author disclosures are available in the supporting information.
Figures
Comment in
-
Beyond the AJR: Updated Guidelines on the Appropriate Use of Amyloid and Tau PET in Clinical Practice.AJR Am J Roentgenol. 2025 Jul;225(1):e2532946. doi: 10.2214/AJR.25.32946. Epub 2025 Mar 19. AJR Am J Roentgenol. 2025. PMID: 40105389 No abstract available.

