Fumarprotocetraric acid and geraniin were identified as novel inhibitors of human respiratory syncytial virus infection in vitro
- PMID: 39776441
- PMCID: PMC11703719
- DOI: 10.3389/fcimb.2024.1484245
Fumarprotocetraric acid and geraniin were identified as novel inhibitors of human respiratory syncytial virus infection in vitro
Abstract
Introduction: Respiratory syncytial virus (RSV) remains a major international public health concern. However, disease treatment is limited to preventive care with monoclonal antibodies and supportive care. In this study, natural products were screened to identify novel anti-RSV inhibitors.
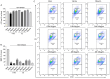
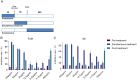
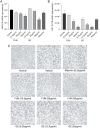
Methods: The antiviral effect of 320 compounds on RSV in HEp-2 cells was tested using a Cytopathic effect (CPE) inhibition assay. The antiviral effect of fumarprotocetraric acid (FUM) and geraniin (GE) were confirmed by Real-time reverse transcription quantitative PCR (Real-time RT-PCR), plaque reduction test, immunofluorescence assay, and Western blot analysis. Real-time PCR was used to detect inflammatory factor expression. ATP assay and JC-1 stain were used to evaluate mitochondrial protection function. The experiment of administration time was used to determine the stages in the RSV life cycle inhibited by FUM and GE. Human metapneumovirus (HMPV) and human rhinovirus (HRV) were used to evaluate the antiviral activities of other respiratory viruses of FUM and GE. Finally, Air-liquid interface human airway epithelium (ALI-HAE) cells were used to evaluate the antiviral effect and mechanism of FUM and GE to RSV.
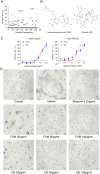
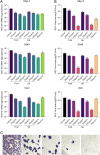
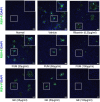
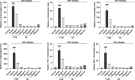
Results: The results showed that FUM and GE can inhibit the replication of RSV in multiple-cell models. Both compounds could dose-dependent inhibit the viral load, RSV nucleic acids level, and RSV-F protein level. Besides, FUM and GE showed good anti-inflammatory activity, mitochondrial protection, and antiviral activity to HMPV and HRV. Meanwhile, our result indicated that FUM and GE can inhibit RSV replication in ALI-HAE cells.
Conclusions: FUM and GE were identified as new inhibitors of RSV infection. At the same time, FUM and GE have anti-inflammatory activity, mitochondrial protection function, and broad-spectrum antiviral activity. These results provide evidence that FUM and GE are potential candidates for the development of novel anti-RSV drugs.
Keywords: antiviral; fumarprotocetraric acid; geraniin; natural products; respiratory syncytial virus.
Copyright © 2024 Wang, Huang, Zhao, Bai, Yan, Du, Zheng and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

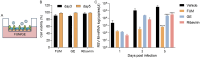
Similar articles
-
Respiratory Syncytial Virus and Human Metapneumovirus Infections in Three-Dimensional Human Airway Tissues Expose an Interesting Dichotomy in Viral Replication, Spread, and Inhibition by Neutralizing Antibodies.J Virol. 2020 Sep 29;94(20):e01068-20. doi: 10.1128/JVI.01068-20. Print 2020 Sep 29. J Virol. 2020. PMID: 32759319 Free PMC article.
-
Preclinical Characterization of PC786, an Inhaled Small-Molecule Respiratory Syncytial Virus L Protein Polymerase Inhibitor.Antimicrob Agents Chemother. 2017 Aug 24;61(9):e00737-17. doi: 10.1128/AAC.00737-17. Print 2017 Sep. Antimicrob Agents Chemother. 2017. PMID: 28652242 Free PMC article.
-
Differential antiviral activities of respiratory syncytial virus (RSV) inhibitors in human airway epithelium.J Antimicrob Chemother. 2018 Jul 1;73(7):1823-1829. doi: 10.1093/jac/dky089. J Antimicrob Chemother. 2018. PMID: 29596680 Free PMC article.
-
[Prospects For the Use of Peptides against Respiratory Syncytial Virus].Mol Biol (Mosk). 2019 Jul-Aug;53(4):541-560. doi: 10.1134/S002689841904013X. Mol Biol (Mosk). 2019. PMID: 31397431 Review. Russian.
-
Respiratory syncytial virus entry inhibitors targeting the F protein.Viruses. 2013 Jan 16;5(1):211-25. doi: 10.3390/v5010211. Viruses. 2013. PMID: 23325327 Free PMC article. Review.
Cited by
-
hMPV Outbreaks: Worldwide Implications of a Re-Emerging Respiratory Pathogen.Microorganisms. 2025 Jun 27;13(7):1508. doi: 10.3390/microorganisms13071508. Microorganisms. 2025. PMID: 40732017 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

