Advances of Stimuli-Responsive Amphiphilic Copolymer Micelles in Tumor Therapy
- PMID: 39776491
- PMCID: PMC11700880
- DOI: 10.2147/IJN.S495387
Advances of Stimuli-Responsive Amphiphilic Copolymer Micelles in Tumor Therapy
Abstract
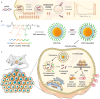
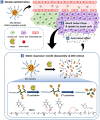
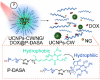
Amphiphilic copolymers are composed of both hydrophilic and hydrophobic chains, which can self-assemble into polymeric micelles in aqueous solution via the hydrophilic/hydrophobic interactions. Due to their unique properties, polymeric micelles have been widely used as drug carriers. Poorly soluble drugs can be covalently attached to polymer chains or non-covalently incorporated in the micelles, with improved pharmacokinetic profiles and enhanced efficacy. In recent years, stimuli-responsive amphiphilic copolymer micelles have attracted significant attention. These micelles can respond to specific stimuli, including physical triggers (light, temperature, etc). chemical stimuli (pH, redox, etc). and physiological factors (enzymes, ATP, etc). Under these stimuli, the structures or properties of the micelles can change, enabling targeted therapy and controlled drug release in tumors. These stimuli-responsive strategies offer new avenues and approaches to enhance the tumor efficacy and reduce drug side effects. We will review the applications of different types of stimuli-responsive amphiphilic copolymer micelles in tumor therapy, aiming to provide valuable guidance for future research directions and clinical translation.
Keywords: delivery system; polymer micelles; stimuli-responsive; tumor therapy.
© 2025 Wang et al.
Conflict of interest statement
The authors declare no conflicts of interest in this work.
Figures



Similar articles
-
pH/redox dual-responsive amphiphilic zwitterionic polymers with a precisely controlled structure as anti-cancer drug carriers.Biomater Sci. 2019 Aug 1;7(8):3190-3203. doi: 10.1039/c9bm00407f. Epub 2019 May 30. Biomater Sci. 2019. PMID: 31145392
-
Functional block copolymer assemblies responsive to tumor and intracellular microenvironments for site-specific drug delivery and enhanced imaging performance.Chem Soc Rev. 2013 Sep 7;42(17):7289-325. doi: 10.1039/c3cs60048c. Epub 2013 Apr 3. Chem Soc Rev. 2013. PMID: 23549663 Review.
-
Polymeric micelles with stimuli-triggering systems for advanced cancer drug targeting.J Drug Target. 2014 Aug;22(7):584-99. doi: 10.3109/1061186X.2014.936872. J Drug Target. 2014. PMID: 25012066 Review.
-
Fabrication of pH- and Ultrasound-Responsive Polymeric Micelles: The Effect of Amphiphilic Block Copolymers with Different Hydrophilic/Hydrophobic Block Ratios for Self-Assembly and Controlled Drug Release.Biomacromolecules. 2025 Apr 14;26(4):2116-2130. doi: 10.1021/acs.biomac.4c01202. Epub 2025 Mar 11. Biomacromolecules. 2025. PMID: 40067950 Free PMC article.
-
Comb-like amphiphilic copolymers bearing acetal-functionalized backbones with the ability of acid-triggered hydrophobic-to-hydrophilic transition as effective nanocarriers for intracellular release of curcumin.Biomacromolecules. 2013 Nov 11;14(11):3973-84. doi: 10.1021/bm401087n. Epub 2013 Oct 29. Biomacromolecules. 2013. PMID: 24107101
Cited by
-
Poly(oligoethylene glycol methylether methacrylate-co-methyl methacrylate) Aggregates as Nanocarriers for Curcumin and Quercetin.Polymers (Basel). 2025 Feb 27;17(5):635. doi: 10.3390/polym17050635. Polymers (Basel). 2025. PMID: 40076126 Free PMC article.
-
Nano-Drug Delivery Systems for Bone Metastases: Targeting the Tumor-Bone Microenvironment.Pharmaceutics. 2025 May 2;17(5):603. doi: 10.3390/pharmaceutics17050603. Pharmaceutics. 2025. PMID: 40430894 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

