The role of Aha1 in cancer and neurodegeneration
- PMID: 39776493
- PMCID: PMC11703849
- DOI: 10.3389/fnmol.2024.1509280
The role of Aha1 in cancer and neurodegeneration
Abstract
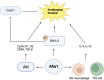
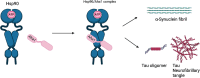
The 90 kDa Heat shock protein (Hsp90) is a family of ubiquitously expressed molecular chaperones responsible for the stabilization and maturation of >400 client proteins. Hsp90 exhibits dramatic conformational changes to accomplish this, which are regulated by partner proteins termed co-chaperones. One of these co-chaperones is called the activator or Hsp90 ATPase activity homolog 1 (Aha1) and is the most potent accelerator of Hsp90 ATPase activity. In conditions where Aha1 levels are dysregulated including cystic fibrosis, cancer and neurodegeneration, Hsp90 mediated client maturation is disrupted. Accumulating evidence has demonstrated that many disease states exhibit large hetero-protein complexes with Hsp90 as the center. Many of these include Aha1, where increased Aha1 levels drive disease states forward. One strategy to block these effects is to design small molecule disruptors of the Hsp90/Aha1 complex. Studies have demonstrated that current Hsp90/Aha1 small molecule disruptors are effective in both models for cancer and neurodegeration.
Keywords: Aha1; Hsp90; cancer; protein–protein interaction; small molecule; tauopathy.
Copyright © 2024 Blagg and Catalfano.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Biological and structural basis for Aha1 regulation of Hsp90 ATPase activity in maintaining proteostasis in the human disease cystic fibrosis.Mol Biol Cell. 2010 Mar 15;21(6):871-84. doi: 10.1091/mbc.e09-12-1017. Epub 2010 Jan 20. Mol Biol Cell. 2010. PMID: 20089831 Free PMC article.
-
p23 and Aha1: Distinct Functions Promote Client Maturation.Subcell Biochem. 2023;101:159-187. doi: 10.1007/978-3-031-14740-1_6. Subcell Biochem. 2023. PMID: 36520307 Review.
-
Aha1 regulates Hsp90's conformation and function in a stoichiometry-dependent way.Biophys J. 2023 Sep 5;122(17):3458-3468. doi: 10.1016/j.bpj.2023.07.020. Epub 2023 Jul 27. Biophys J. 2023. PMID: 37515325 Free PMC article.
-
Silencing of HSP90 cochaperone AHA1 expression decreases client protein activation and increases cellular sensitivity to the HSP90 inhibitor 17-allylamino-17-demethoxygeldanamycin.Cancer Res. 2008 Feb 15;68(4):1188-97. doi: 10.1158/0008-5472.CAN-07-3268. Cancer Res. 2008. PMID: 18281495
-
p23 and Aha1.Subcell Biochem. 2015;78:113-31. doi: 10.1007/978-3-319-11731-7_6. Subcell Biochem. 2015. PMID: 25487019 Review.
References
-
- Baker-Williams A. J., Hashmi F., Budzyński M. A., Woodford M. R., Gleicher S., Himanen S. V., et al. . (2019). Co-chaperones TIMP2 and AHA1 competitively regulate extracellular HSP90:client MMP2 activity and matrix proteolysis. Cell Rep. 28, 1894–1906.e6. doi: 10.1016/j.celrep.2019.07.045, PMID: - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

