Deer antler reserve mesenchyme cells modified with miR-145 promote chondrogenesis in cartilage regeneration
- PMID: 39776601
- PMCID: PMC11705092
- DOI: 10.3389/fvets.2024.1500969
Deer antler reserve mesenchyme cells modified with miR-145 promote chondrogenesis in cartilage regeneration
Abstract
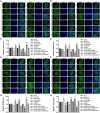
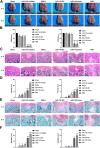
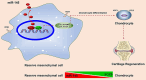
Deer antler-derived reserve mesenchyme cells (RMCs) are a promising source of cells for cartilage regeneration therapy due to their chondrogenic differentiation potential. However, the regulatory mechanism has not yet been elucidated. In this study, we analyzed the role of microRNAs (miRNAs) in regulating the differentiation of RMCs and in the post-transcriptional regulation of chondrogenesis and hypertrophic differentiation at the molecular and histological levels. The results showed that RMCs showed typical MSC differentiation potentials. During chondrogenic differentiation, we obtained the expression profile of miRNAs, among which miR- 145 was the most prominent candidate as a key microRNA involved in the balance of chondral and endochondral differentiation. Knockdown of miR-145 promoted chondrogenesis and inhibited hypertrophy differentiation in RMCs. Mechanically, by prediction through online databases combined with dual-luciferase reporter assay, SOX9 was suggested as a target of miR-145. Further validation experiments confirmed that knockdown of miR-145 contributed to the balance between endochondral versus chondral differentiation of RMCs by targeting SOX9. Additionally, RMCs transfected with the miR-145-knockdown-mediated lentiviral vector successfully promoted cartilage regeneration in vivo. In summary, our study suggested that the reciprocal negative feedback between SOX9 and miR-145 was essential for balancing between endochondral versus chondral differentiation of RMCs. Our study suggested that modification of RMCs using miRNAs transduction might be an effective treatment for cartilage defects.
Keywords: SOX9; cartilage regeneration; chondrogenesis; miR-145; reserve mesenchyme cells.
Copyright © 2024 Jia, Han, Li, Zhang, Ma, Wang, Wang, Yan, Li, Shen, Chen, Li, Zhang, Hu and Du.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






Similar articles
-
MiR-218 affects hypertrophic differentiation of human mesenchymal stromal cells during chondrogenesis via targeting RUNX2, MEF2C, and COL10A1.Stem Cell Res Ther. 2020 Dec 10;11(1):532. doi: 10.1186/s13287-020-02026-6. Stem Cell Res Ther. 2020. PMID: 33303006 Free PMC article.
-
MicroRNA-27b targets CBFB to inhibit differentiation of human bone marrow mesenchymal stem cells into hypertrophic chondrocytes.Stem Cell Res Ther. 2020 Sep 11;11(1):392. doi: 10.1186/s13287-020-01909-y. Stem Cell Res Ther. 2020. PMID: 32917285 Free PMC article.
-
Reciprocal negative feedback between Prrx1 and miR-140-3p regulates rapid chondrogenesis in the regenerating antler.Cell Mol Biol Lett. 2024 Apr 20;29(1):56. doi: 10.1186/s11658-024-00573-x. Cell Mol Biol Lett. 2024. PMID: 38643083 Free PMC article.
-
Regulation and function of SOX9 during cartilage development and regeneration.Semin Cancer Biol. 2020 Dec;67(Pt 1):12-23. doi: 10.1016/j.semcancer.2020.04.008. Epub 2020 May 4. Semin Cancer Biol. 2020. PMID: 32380234 Review.
-
New physiological insights into the phenomena of deer antler: A unique model for skeletal tissue regeneration.J Orthop Translat. 2020 Dec 24;27:57-66. doi: 10.1016/j.jot.2020.10.012. eCollection 2021 Mar. J Orthop Translat. 2020. PMID: 33437638 Free PMC article. Review.
Cited by
-
Mitigating Health Risks Through Environmental Tracking of Pseudomonas aeruginosa.Curr Microbiol. 2024 Dec 24;82(1):57. doi: 10.1007/s00284-024-04036-6. Curr Microbiol. 2024. PMID: 39718600 Review.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

