Discoidin Domain Receptor 1 impacts bone microarchitecture with aging in female mice
- PMID: 39776614
- PMCID: PMC11701535
- DOI: 10.1093/jbmrpl/ziae160
Discoidin Domain Receptor 1 impacts bone microarchitecture with aging in female mice
Abstract
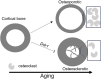
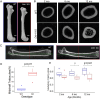
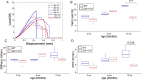
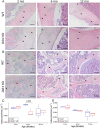
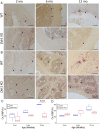
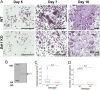
Discoidin Domain Receptor 1 (DDR1) is a receptor tyrosine kinase that binds to and is activated by collagen(s), including collagen type I. Ddr1 deletion in osteoblasts and chondrocytes has previously demonstrated the importance of this receptor in bone development. In this study, we examined the effect of DDR1 ablation on bone architecture and mechanics as a function of aging. Femurs were collected from female global Ddr1 knockout (KO) and wild-type (WT) mice at 2, 6, and 12 mo of age and analyzed by high-resolution micro-computed tomography (μCT), mechanical testing, and histology. Primary monocytes were collected for in vitro osteoclastogenesis assays. Our studies on younger (2 mo) mice revealed no significant differences between the two genotypes and the microarchitectural and mechanical features had a similar trend as those reported earlier for osteoblast or chondrocyte specific Ddr1 knockdown. At an advanced age (12 mo), significant differences were noted across the two genotypes. μCT analysis showed a decrease in medullary cavity area as well as increased trabeculation in cortical and trabecular bone in the Ddr1 KO vs. WT mice. In addition, Ddr1 KO mouse bones exhibited reduced mechanical properties (lower peak load, yield load, and energy to yield) at 12 mo. Histological analysis revealed reduced osteoclast count in Ddr1 KO femurs at 12 mo with no significant difference in osteocyte count between the genotypes. In vitro, osteoclastogenesis was impaired in Ddr1 KO bone marrow derived cells. These results suggest that DDR1 deficiency adversely impacts osteoclast differentiation and bone remodeling in an age-dependent manner.
Keywords: aging; biomechanics; bone microct; genetic animal model; osteoclasts.
© The Author(s) 2024. Published by Oxford University Press on behalf of the American Society for Bone and Mineral Research.
Conflict of interest statement
None declared.
Figures

References
-
- Dempster D, Felsenberg D, Geest S, eds. The Bone Quality Book: A Guide to Factors Influencing Bone Strength. Excerpta Medica Elsevier; 2006.
LinkOut - more resources
Full Text Sources
Research Materials

