Crystal structure of catena-poly[bis-(N, O-di-methyl-hydroxyl-ammonium) [di-μ-bromido-di-bromido-stannate(II)]]
- PMID: 39776634
- PMCID: PMC11701764
- DOI: 10.1107/S2056989024012027
Crystal structure of catena-poly[bis-(N, O-di-methyl-hydroxyl-ammonium) [di-μ-bromido-di-bromido-stannate(II)]]
Abstract
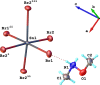
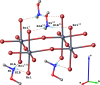
The title compound, {(C2H8NO)2[SnBr4]} n , is a layered hybrid perovskite crystallizing in the monoclinic space group C2/c. The asymmetric unit consists of one H3C-O-NH2 +-CH3 cation (Me2HA+), one SnII atom located on a twofold rotation axis, and two Br atoms. The SnII atom has a distorted octa-hedral coordination environment formed by the bromido ligands. The {SnBr6} units corner-share their equatorial Br atoms, forming infinite mono-layers that extend parallel to the ab plane. These inorganic layers are sandwiched by the organic Me2HA+ cations organized in double-layers; stacking of the layers is along the c-axis direction. Consecutive inorganic layers, separated by the organic cations, are shifted relative to each other along the b-axis direction. Specifically, the SnII atom in one inorganic layer is offset by 3.148 Å along the b axis relative to the SnII atom in an adjacent inorganic layer. The N,O-di-methyl-hydroxyl-ammonium cation forms two hydrogen bonds with the axial bromide anions of the inorganic layers as acceptors, and leads to the cohesion of the crystal structure. According to Hirshfeld surface analysis, the highest contributions to the crystal packing are from H⋯H (46.2%), Br⋯H (38.5%), and H⋯O (14.8%) contacts.
Keywords: Hirshfeld surface analysis; N,O-dimethylhydroxylamine; crystal structure; metal halides; stannate; tin(II) bromide.
© Sirenko et al. 2025.
Figures
 + x,
+ x,  + y, z; (ii) 1 − x, y,
+ y, z; (ii) 1 − x, y,  − z; (iii)
− z; (iii)  − x,
− x,  + y,
+ y,  − z.]
− z.]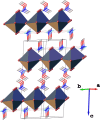
 − z; (ii)
− z; (ii)  − x,
− x,  + y,
+ y,  − z; (iii) −
− z; (iii) − + x, −
+ x, − + y, z.]
+ y, z.]
 + z.]
+ z.]
References
-
- Azmi, R., Zhumagali, S., Bristow, H., Zhang, S., Yazmaciyan, A., Pininti, A. R., Utomo, D. S., Subbiah, A. S. & De Wolf, S. (2024). Adv. Mater.36, e2211317. - PubMed
-
- Ban, I., Kristl, M., Volavšek, B. & Golič, L. (1999). Monatsh. Chem.130, 401–408.
-
- Ben Hmida, W., Jellali, A., Abid, H., Hamdi, B., Naili, H. & Zouari, R. (2019). J. Mol. Struct.1184, 604–614.
-
- Clark, R. C. & Reid, J. S. (1995). Acta Cryst. A51, 887–897.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
