Determination of clostebol residues in the urine of slaughter animals using liquid chromatography-tandem mass spectrometry
- PMID: 39776689
- PMCID: PMC11702253
- DOI: 10.2478/jvetres-2024-0070
Determination of clostebol residues in the urine of slaughter animals using liquid chromatography-tandem mass spectrometry
Abstract
Introduction: Synthetic anabolic hormones, which may pose a potential risk to human health, should not be used in fattening food-producing animals. Because of the hormonal effects they cause, growth-promoting compounds are banned by legislation in the EU for use in animal husbandry. Consequently, all EU member states are required to conduct monitoring tests on the content and residues of these compounds in prescribed biological matrices to ensure the safety of food consumers. The aim of this research was to develop a liquid chromatography-tandem mass spectrometry method for the detection of the residue of one such anabolic hormone, clostebol in food animal urine.
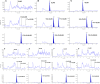
Material and methods: Clostebol and its marker metabolite residues were determined by a method involving enzymatic hydrolysis, isolation of compounds from urine on a C18 solid-phase extraction (SPE) column, purification of the extract by liquid-liquid extraction using n-pentane and a NH2 SPE column, and detection by liquid chromatography-tandem mass spectrometry.
Results: No traces of this anabolic steroid hormone or its metabolites were found in any of the samples tested. The method was validated in accordance with the current requirements for confirmatory methods, and the determined parameters of the decision limits necessary for assessing sample compliance met the specified criteria.
Conclusion: In 2023, the method was introduced for testing under the National Control Plan in Poland. Up to July 19, 2024, 53 urine samples from different animal species had been tested.
Keywords: LC-MS/MS; clostebol; hormone residue; urine.
© 2024 Iwona Matraszek-Żuchowska et al., published by Sciendo.
Conflict of interest statement
Conflict of Interests Statement: The authors declare that there are no conflict of interests regarding the publication of this article.
Figures
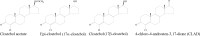
References
-
- Baglai A., Blokland M.H., Mol H.G.J., Gargano A.F.G., Van der Wal S., Schoenmakers P.J. Enhancing detectability of anabolic-steroid residues in bovine urine by actively modulated online comprehensive two-dimensional liquid chromatography – high resolution mass spectrometry Anal Chim Acta. 2018;1013:87. doi: 10.1016/j.aca.2017.12.043. : . , , –. , doi: . - DOI - PubMed
-
- Council of the European Union Council Directive 96/22/EC of 29 April 1996, concerning the prohibition on the use in stockfarming of certain substances having a hormonal or thyreostatic action and of β-agonists, and repealing Directives 81/602/EEC, 88/146/EEC and 88/299/EEC OJEC L. 1996;125:39. http://data.europa.eu/eli/dir/1996/22/oj : . , , , 23/05/1996, 3–9*, .
-
- Crabbe P., Meyer U.J., Zhi Z.L., Pieraccini G., O’Keeffe M., Van Peteghem C. Screening of Clostebol and its metabolites in Bovine Urine with ELISA and Comparison with GC-MS Results in an Interlaboratory Study J Anal Toxicol. 2003;27:213. https://academic.oup.com/jat/article/27/4/213/721348 : . , , –. , . - PubMed
-
- Crabbe P., Pieraccini G., Bartolucci G., Moneti G., Van Peteghem C. Influence of Helix pomatia Enzyme Preparations on the Oxidative Conversion of Some Clostebol Acetate metabolites in Urine J Anal Toxicol. 2002;26:73. https://academic.oup.com/jat/article/26/2/73/739248 : . , , –. , . - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
