Bisphosphonate-mineralized nano-IFNγ suppresses residual tumor growth caused by incomplete radiofrequency ablation through metabolically remodeling tumor-associated macrophages
- PMID: 39776793
- PMCID: PMC11700868
- DOI: 10.7150/thno.100998
Bisphosphonate-mineralized nano-IFNγ suppresses residual tumor growth caused by incomplete radiofrequency ablation through metabolically remodeling tumor-associated macrophages
Abstract
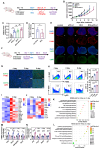
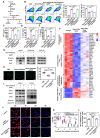
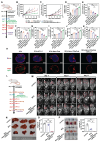
Rationale: Radiofrequency ablation (RFA), as a minimally invasive surgery strategy based on local thermal-killing effect, is widely used in the clinical treatment of multiple solid tumors. Nevertheless, RFA cannot achieve the complete elimination of tumor lesions with larger burden or proximity to blood vessels. Incomplete RFA (iRFA) has even been validated to promote residual tumor growth due to the suppressive tumor immune microenvironment (TIME). Therefore, exploring strategies to remodel TIME is a key issue for the development of RFA therapy. Methods: The negative effect of iRFA on colorectal cancer therapy was firstly investigated. Then a zoledronate-mineralized nanoparticle loaded with IFNγ (Nano-IFNγ/Zole) was designed and its tumor suppressive efficacy was evaluated. Finally, the metabolic reprogramming mechanism of Nano-IFNγ/Zole on tumor-associated macrophages (TAMs) was studied in detail. Results: We found iRFA dynamically altered TIME and promoted TAM differentiation from M1 to M2. Nano-IFNγ/Zole was fabricated to metabolically remodel TAMs. IFNγ in Nano-IFNγ/Zole concentrated in the ablation site to play a long-term remodeling role. Acting on mevalonate pathway, Nano-IFNγ/Zole was discovered to reduce lysosomal acidification and activate transcription factor TFEB by inhibiting isoprene modification of the Rab protein family. These mechanisms, in conjunction with IFNγ-activated JAK/STAT1 signaling, accelerated the reprogramming of TAMs from M2 to M1, and suppressed tumor recurrence after iRFA. Conclusions: This study elaborates the synergistic mechanism of zoledronate and IFNγ in Nano-IFNγ/Zole to reshape suppressive TIME caused by iRFA by remodeling TAMs, and highlights the important value of metabolically induced cellular reprogramming. Since both zoledronate and IFNγ have already been approved in clinics, this integrative nano-drug delivery system establishes an effective strategy with great translational promise to overcome the poor prognosis after clinically incomplete RFA.
Keywords: interferon-γ; mevalonate metabolic pathway; radiofrequency ablation; tumor immune microenvironment; tumor-associated macrophages.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures




Similar articles
-
oHSV2-mGM repolarizes TAMs and cooperates with αPD1 to reprogram the immune microenvironment of residual cancer after radiofrequency ablation.Biomed Pharmacother. 2024 Sep;178:117060. doi: 10.1016/j.biopha.2024.117060. Epub 2024 Jul 24. Biomed Pharmacother. 2024. PMID: 39053421
-
Tumor microenvironment remodeling and tumor therapy based on M2-like tumor associated macrophage-targeting nano-complexes.Theranostics. 2021 Jan 1;11(6):2892-2916. doi: 10.7150/thno.50928. eCollection 2021. Theranostics. 2021. PMID: 33456579 Free PMC article.
-
STING agonist-based hydrogel enhances immune activation in synergy with radiofrequency ablation for hepatocellular carcinoma treatment.J Control Release. 2024 May;369:296-308. doi: 10.1016/j.jconrel.2024.01.048. Epub 2024 Mar 30. J Control Release. 2024. PMID: 38301925
-
Radiofrequency ablation: mechanisms and clinical applications.MedComm (2020). 2024 Oct 2;5(10):e746. doi: 10.1002/mco2.746. eCollection 2024 Oct. MedComm (2020). 2024. PMID: 39359691 Free PMC article. Review.
-
Targeting lipid metabolism of macrophages: A new strategy for tumor therapy.J Adv Res. 2025 Feb;68:99-114. doi: 10.1016/j.jare.2024.02.009. Epub 2024 Feb 18. J Adv Res. 2025. PMID: 38373649 Free PMC article. Review.
Cited by
-
Nanomaterials in the diagnosis and treatment of gastrointestinal tumors: New clinical choices and treatment strategies.Mater Today Bio. 2025 Apr 19;32:101782. doi: 10.1016/j.mtbio.2025.101782. eCollection 2025 Jun. Mater Today Bio. 2025. PMID: 40331152 Free PMC article. Review.
-
Global Research Trends and Hotspots in the Role of Cholesterol in Colorectal Cancer: A Bibliometric Analysis.Hum Mutat. 2025 May 24;2025:6546114. doi: 10.1155/humu/6546114. eCollection 2025. Hum Mutat. 2025. PMID: 40452858 Free PMC article.
-
Multifaceted Applications of Nanomaterials in Colorectal Cancer Management: Screening, Diagnostics, and Therapeutics.Int J Nanomedicine. 2025 Jun 10;20:7271-7294. doi: 10.2147/IJN.S520616. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40520059 Free PMC article. Review.
References
-
- Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer. 2014;14:199–208. - PubMed
-
- Chen M, Peng Z, Xu L, Zhang Y, Liang H, Li J. Role of radiofrequency ablation in the treatment of hepatocellular carcinoma: experience of a cancer center in China. Oncology. 2011;81:100–104. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

