Ex vivo enhancement of CD8+ T cell activity using functionalized hydrogel encapsulating tonsil-derived lymphatic endothelial cells
- PMID: 39776798
- PMCID: PMC11700866
- DOI: 10.7150/thno.100079
Ex vivo enhancement of CD8+ T cell activity using functionalized hydrogel encapsulating tonsil-derived lymphatic endothelial cells
Abstract
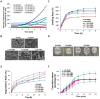
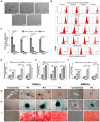
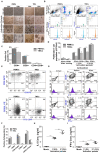
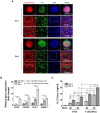
Rationale: This study investigates a method for programming immune cells using a biomaterial-based system, providing an alternative to traditional ex vivo cell manipulation techniques. It addresses the limitations of engineered adoptive T cell therapies, such as T cell exhaustion, by introducing a gelatin-hyaluronic acid (GH-GMA) hydrogel system. Methods: We characterized tonsil mesenchymal stem cells (TMSCs), lymphatic endothelial cells (T-LECs), stimulated T-CD8+ T cells (STCs), and GH-GMA biomaterials. The 10% 5:1 GH-GMA hydrogel, loaded with anti-CD28, cytokines interleukin-2 (IL-2) and vascular endothelial growth factor C (VEGF-C), forms a functional hydrogel capable of releasing these immune-stimulating factors. T-LEC spheroids, derived from tonsil mesenchymal stem cells (TMSCs), were encapsulated within the hydrogel to act as antigen-presenting cells for T cells. Results: Co-encapsulation of STCs and T-LEC spheroids in the functional hydrogel resulted in significant expansion and enrichment of STCs during cultivation. Moreover, when cancer cells were co-encapsulated with STCs and T-LECs, there was increased migration of STCs towards the cancer cells and elevated expression of PD-L1 on the cancer cells. Conclusions: These findings suggest that the GH-GMA hydrogel, combined with anti-CD28, IL-2, VEGF-C, and T-LEC spheroids, enhances T cell activity, presenting a promising platform for cancer immunotherapies and modulation of the suppressive tumor microenvironment.
Keywords: Cancer immunology; Gelatin-hyaluronic acid based hydrogel; T cell activation; Tonsil derived CD8+ T cells; Tonsil derived lymphatic endothelial cells.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



Similar articles
-
Chondrogenically primed tonsil-derived mesenchymal stem cells encapsulated in riboflavin-induced photocrosslinking collagen-hyaluronic acid hydrogel for meniscus tissue repairs.Acta Biomater. 2017 Apr 15;53:318-328. doi: 10.1016/j.actbio.2017.01.081. Epub 2017 Feb 1. Acta Biomater. 2017. PMID: 28161573
-
Injectable Hydrogels Based on Hyaluronic Acid and Gelatin Combined with Salvianolic Acid B and Vascular Endothelial Growth Factor for Treatment of Traumatic Brain Injury in Mice.Molecules. 2024 Apr 10;29(8):1705. doi: 10.3390/molecules29081705. Molecules. 2024. PMID: 38675525 Free PMC article.
-
Sustained release of parathyroid hormone via in situ cross-linking gelatin hydrogels improves the therapeutic potential of tonsil-derived mesenchymal stem cells for hypoparathyroidism.J Tissue Eng Regen Med. 2018 Mar;12(3):e1747-e1756. doi: 10.1002/term.2430. Epub 2017 Jun 27. J Tissue Eng Regen Med. 2018. PMID: 28244684
-
Hydrogel elasticity and microarchitecture regulate dental-derived mesenchymal stem cell-host immune system cross-talk.Acta Biomater. 2017 Sep 15;60:181-189. doi: 10.1016/j.actbio.2017.07.017. Epub 2017 Jul 12. Acta Biomater. 2017. PMID: 28711686 Free PMC article.
-
Extracellular vesicle-loaded hydrogels for tissue repair and regeneration.Mater Today Bio. 2022 Dec 21;18:100522. doi: 10.1016/j.mtbio.2022.100522. eCollection 2023 Feb. Mater Today Bio. 2022. PMID: 36593913 Free PMC article. Review.
Cited by
-
Nanostrategies synergize with locoregional interventional therapies for boosting antitumor immunity.Bioact Mater. 2025 May 31;51:634-649. doi: 10.1016/j.bioactmat.2025.05.016. eCollection 2025 Sep. Bioact Mater. 2025. PMID: 40521175 Free PMC article.
References
-
- Altman A, Coggeshall KM, Mustelin T. Molecular events mediating T cell activation. Adv Immunol. 1990;48:227–360. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

