Vascularized tumor on a microfluidic chip to study mechanisms promoting tumor neovascularization and vascular targeted therapies
- PMID: 39776800
- PMCID: PMC11700857
- DOI: 10.7150/thno.95334
Vascularized tumor on a microfluidic chip to study mechanisms promoting tumor neovascularization and vascular targeted therapies
Abstract
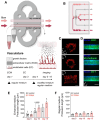
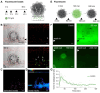
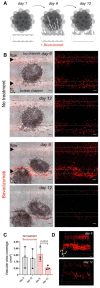
The cascade of events leading to tumor formation includes induction of a tumor supporting neovasculature, as a primary hallmark of cancer. Developing vasculature is difficult to evaluate in vivo but can be captured using microfluidic chip technology and patient derived cells. Herein, we established an on chip approach to investigate the mechanisms promoting tumor vascularization and vascular targeted therapies via co-culture of cancer spheroids and endothelial cells in a three dimensional environment. Methods: We investigated both, tumor neovascularization and therapy, via co-culture of human derived endothelial cells and adjacently localized metastatic renal cell carcinoma spheroids on a commercially available microfluidic chip system. Metastatic renal cell carcinoma spheroids adjacent to primary vessels model tumor, and induce vessels to sprout neovasculature towards the tumor. We monitored real time changes in vessel formation, probed the interactions of tumor and endothelial cells, and evaluated the role of important effectors in tumor vasculature. In addition to wild type endothelial cells, we evaluated endothelial cells that overexpress Prostate Specific Membrane Antigen (PSMA), that has emerged as a marker of tumor associated neovasculature. We characterized the process of neovascularization on the microfluidic chip stimulated by enhanced culture medium and the investigated metastatic renal cell carcinomas, and assessed endothelial cells responses to vascular targeted therapy with bevacizumab via confocal microscopy imaging. To emphasize the potential clinical relevance of metastatic renal cell carcinomas on chip, we compared therapy with bevacizumab on chip with an in vivo model of the same tumor. Results: Our model permitted real-time, high-resolution observation and assessment of tumor-induced angiogenesis, where endothelial cells sprouted towards the tumor and mimicked a vascular network. Bevacizumab, an antiangiogenic agent, disrupted interactions between vessels and tumors, destroying the vascular network. The on chip approach enabled assessment of endothelial cell biology, vessel's functionality, drug delivery, and molecular expression of PSMA. Conclusion: Observations in the vascularized tumor on chip permitted direct and conclusive quantification of vascular targeted therapies in weeks as opposed to months in a comparable animal model, and bridged the gap between in vitro and in vivo models.
Keywords: confocal imaging; microfluidic chip; neovascularization; optoacoustic imaging; targeted therapies.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


Update of
-
Vascularized tumor on a microfluidic chip to study mechanisms promoting tumor neovascularization and vascular targeted therapies.bioRxiv [Preprint]. 2023 Aug 8:2023.08.07.552309. doi: 10.1101/2023.08.07.552309. bioRxiv. 2023. Update in: Theranostics. 2025 Jan 1;15(3):766-783. doi: 10.7150/thno.95334. PMID: 37609216 Free PMC article. Updated. Preprint.
References
-
- Hanahan D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022;12(1):31–46. - PubMed
-
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249–57. - PubMed
-
- Sharma SV, Haber DA, Settleman J. Cell line-based platforms to evaluate the therapeutic efficacy of candidate anticancer agents. Nat Rev Cancer. 2010;10(4):241–53. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

