Monitoring of cancer ferroptosis with [18F]hGTS13, a system xc- specific radiotracer
- PMID: 39776801
- PMCID: PMC11700874
- DOI: 10.7150/thno.101882
Monitoring of cancer ferroptosis with [18F]hGTS13, a system xc- specific radiotracer
Abstract
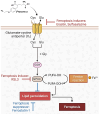
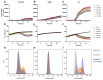
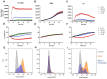
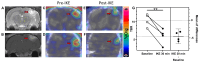
Glioblastoma multiforme (GBM) is the most common and aggressive primary brain tumor in adults, characterized by resistance to conventional therapies and poor survival. Ferroptosis, a form of regulated cell death driven by lipid peroxidation, has recently emerged as a promising therapeutic target for GBM treatment. However, there are currently no non-invasive imaging techniques to monitor the engagement of pro-ferroptotic compounds with their respective targets, or to monitor the efficacy of ferroptosis-based therapies. System xc-, an important player in cellular redox homeostasis, plays a critical role in ferroptosis by mediating the exchange of cystine for glutamate, thus regulating the availability of cysteine, a crucial precursor for glutathione synthesis, and influencing the cellular antioxidant defense system. We have recently reported the development and validation of [18F]hGTS13, a radiopharmaceutical specific for system xc-. Methods: In the current work, we characterized the sensitivity of various cell lines to pro-ferroptotic compounds and evaluated the ability of [18F]hGTS13 to distinguish between sensitive and resistant cell lines and monitor changes in response to ferroptosis-inducing investigational compounds. We then associated changes in [18F]hGTS13 uptake with cellular glutathione content. Furthermore, we evaluated [18F]hGTS13 uptake in a rat model of glioma, both before and after treatment with imidazole ketone erastin (IKE), a pro-ferroptotic inhibitor of system xc- activity. Results: Treatment with erastin2, a system xc- inhibitor, significantly decreased [18F]hGTS13 uptake and cellular glutathione content in vitro. Dynamic PET/CT imaging of C6 glioma-bearing rats with [18F]hGTS13 revealed high and sustained uptake within the intracranial glioma and this uptake was decreased upon pre-treatment with IKE. Conclusion: In summary, [18F]hGTS13 represents a promising tool to distinguish cell types that demonstrate sensitivity or resistance to ferroptosis-inducing therapies that target system xc-, and monitor the engagement of these drugs.
Keywords: PET imaging; [18F]hGTS13; ferroptosis; system xc-.
© The author(s).
Conflict of interest statement
Competing Interests: AM, LR, and CB have filed a provisional application for the method of using system xc- radiotracers in the context of cancer ferroptosis. The authors otherwise declare no conflicts of interest.
Figures



References
-
- Liang C, Zhang X, Yang M, Dong X. Recent progress in ferroptosis inducers for cancer therapy. Adv Mater. 2019;31:1904197. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

