Protein serine/threonine phosphatases in tumor microenvironment: a vital player and a promising therapeutic target
- PMID: 39776803
- PMCID: PMC11700861
- DOI: 10.7150/thno.104529
Protein serine/threonine phosphatases in tumor microenvironment: a vital player and a promising therapeutic target
Abstract
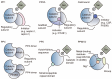
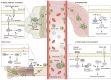
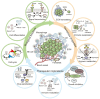
The tumor microenvironment (TME) is involved in cancer initiation and progression. With advances in the TME field, numerous therapeutic approaches, such as antiangiogenic treatment and immune checkpoint inhibitors, have been inspired and developed. Nevertheless, the sophisticated regulatory effects on the biological balance of the TME remain unclear. Decoding the pathological features of the TME is urgently needed to understand the tumor ecosystem and develop novel antitumor treatments. Protein serine/threonine phosphatases (PSPs) are responsible for inverse protein phosphorylation processes. Aberrant expression and dysfunction of PSPs disturb cellular homeostasis, reprogram metabolic processes and reshape the immune landscape, thereby contributing to cancer progression. Some therapeutic implications, such as the use of PSPs as targets, have drawn the attention of researchers and clinicians. To date, the effects of PSP inhibitors are less satisfactory in real-world practice. With breakthroughs in sequencing technologies, scientists can decipher TME investigations via multiomics and higher resolution. These benefits provide an opportunity to explore the TME in a more comprehensive manner and inspire more findings concerning PSPs in the TME. The current review starts by introducing the canonical knowledge of PSPs, including their members, structures and posttranslational modifications for activities. We then summarize the functions of PSPs in regulating cellular homeostasis. In particular, we specified the up-to-date roles of PSPs in modulating the immune microenvironment, adopting hypoxia, reprogramming metabolic processes, and responding to extracellular matrix remodeling. Finally, we introduce preclinical PSP inhibitors with translational value and conclude with clinical trials of PSP inhibitors for cancer treatment.
Keywords: PSP superfamily; PSPs inhibitors; clinical translation; immune therapy; tumor microenvironment.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


Similar articles
-
Tumor microenvironment: recent advances in understanding and its role in modulating cancer therapies.Med Oncol. 2025 Mar 18;42(4):117. doi: 10.1007/s12032-025-02641-4. Med Oncol. 2025. PMID: 40102282 Review.
-
Tumor metabolic regulators: key drivers of metabolic reprogramming and the promising targets in cancer therapy.Mol Cancer. 2025 Jan 9;24(1):7. doi: 10.1186/s12943-024-02205-6. Mol Cancer. 2025. PMID: 39789606 Free PMC article. Review.
-
Taking a Full Snapshot of Cancer Biology: Deciphering the Tumor Microenvironment for Effective Cancer Therapy in the Oncology Clinic.OMICS. 2020 Apr;24(4):175-179. doi: 10.1089/omi.2020.0019. Epub 2020 Mar 13. OMICS. 2020. PMID: 32176591 Review.
-
The updated landscape of tumor microenvironment and drug repurposing.Signal Transduct Target Ther. 2020 Aug 25;5(1):166. doi: 10.1038/s41392-020-00280-x. Signal Transduct Target Ther. 2020. PMID: 32843638 Free PMC article. Review.
-
Role of the tumor microenvironment in cancer therapy: unveiling new targets to overcome drug resistance.Med Oncol. 2025 May 7;42(6):202. doi: 10.1007/s12032-025-02754-w. Med Oncol. 2025. PMID: 40332723 Review.
References
-
- Brautigan DL. Flicking the switches: phosphorylation of serine/threonine protein phosphatases. Semin Cancer Biol. 1995;6:211–7. - PubMed
-
- Brautigan DL, Shenolikar S. Protein Serine/Threonine Phosphatases: Keys to Unlocking Regulators and Substrates. Annu Rev Biochem. 2018;87:921–64. - PubMed
-
- Seifried A, Schultz J, Gohla A. Human HAD phosphatases: structure, mechanism, and roles in health and disease. FEBS J. 2013;280:549–71. - PubMed
-
- Ceulemans H, Bollen M. Functional diversity of protein phosphatase-1, a cellular economizer and reset button. Physiol Rev. 2004;84:1–39. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

