OTUB2 contributes to vascular calcification in chronic kidney disease via the YAP-mediated transcription of PFKFB3
- PMID: 39776804
- PMCID: PMC11700865
- DOI: 10.7150/thno.98660
OTUB2 contributes to vascular calcification in chronic kidney disease via the YAP-mediated transcription of PFKFB3
Abstract
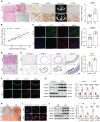
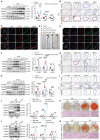
Rationale: Chronic kidney disease (CKD) is a global public health issue, with vascular calcification (VC) being a common and deadly complication. Despite its prevalence, the underlying mechanisms of VC remain unclear. In this study, we aimed to investigate whether and how Otubain-2 (OTUB2) contributes to VC. Methods: The relationship between OTUB2 and VC was examined via immunohistochemical and immunofluorescence staining of discarded calcified radial arteries from uremic patients who underwent arteriovenous fistula operations. Additionally, mice were fed a 0.2% adenine diet supplemented with 1.2% phosphorus to establish a model of CKD-related VC. Vascular smooth muscle cell (VSMC)-specific OTUB2 knockout and overexpression were performed in vivo via the delivery of adeno-associated virus 9 vectors to manipulate the expression of OTUB2. Additionally, a calcified VSMC model was established to explore the roles of OTUB2 in VC by evaluating changes in osteogenic marker expression and calcium deposition. Results: Our results revealed a significant upregulation of OTUB2 expression during VC progression. OTUB2 overexpression upregulated the expression of osteogenic markers and exacerbated VSMC calcification, as verified by Von Kossa and Alizarin red staining. Conversely, VSMC-specific OTUB2 deficiency significantly mitigated adenine diet-induced VC in CKD mice. OTUB2 knockdown or inhibition decreased Yes-associated protein (YAP) abundance. Mechanistically, OTUB2 bound to YAP, decreasing its K48-linked polyubiquitination and inhibiting its subsequent degradation. Knockdown or inhibition of YAP abolished the effect of OTUB2 overexpression on VSMC calcification, indicating a YAP-mediated mechanism. Furthermore, the YAP/TEAD1 complex bound to the promoter of PFKFB3, increasing its transcriptional activity, as determined by CUT&RUN-qPCR. The knockdown or inhibition of PFKFB3 alleviated the procalcific effects of OTUB2. Conclusions: Our findings indicate that OTUB2 promotes VC at least partially by activating the YAP-PFKFB3 signaling pathway. Targeting OTUB2 may be an appealing therapeutic strategy for VC.
Keywords: OTUB2; YAP.; chronic kidney disease; ubiquitin-proteasome degradation; vascular calcification.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures





References
-
- Li X, Liu A, Xie C, Chen Y, Zeng K, Xie C. et al. The transcription factor GATA6 accelerates vascular smooth muscle cell senescence-related arterial calcification by counteracting the role of anti-aging factor SIRT6 and impeding DNA damage repair. Kidney Int. 2024;105(1):115–131. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

