Perfluorocarbon-polyepinephrine core-shell nanoparticles as a near-infrared light activatable theranostic platform for bimodal imaging-guided photothermal/chemodynamic synergistic cancer therapy
- PMID: 39776810
- PMCID: PMC11700858
- DOI: 10.7150/thno.102743
Perfluorocarbon-polyepinephrine core-shell nanoparticles as a near-infrared light activatable theranostic platform for bimodal imaging-guided photothermal/chemodynamic synergistic cancer therapy
Abstract
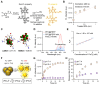
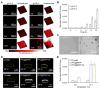
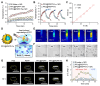
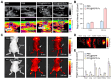
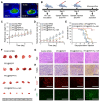
Background: Activatable multifunctional nanoparticles present considerable advantages in cancer treatment by integrating both diagnostic and therapeutic functionalities into a single platform. These nanoparticles can be precisely engineered to selectively target cancer cells, thereby reducing the risk of damage to healthy tissues. Once localized at the target site, they can be activated by external stimuli such as light, pH changes, or specific enzymes, enabling precise control over the release of therapeutic agents or the initiation of therapeutic effects. Furthermore, these nanoparticles can be designed to incorporate multiple therapeutic modalities, including chemotherapy, photothermal therapy (PTT), and chemodynamic therapy (CDT). This comprehensive approach facilitates real-time monitoring of treatment efficacy and allows for dynamic adjustments to therapy, resulting in more personalized and effective cancer treatments. Methods: This study reports the synthesis of perfluorocarbon (PFC)-encapsulated fluorescent polyepinephrine (PEPP) nanoshells chelated with Fe2+ (PFC@PEPP-Fe) and explores their potential for bimodal imaging and synergistic combination therapy in cancer treatment. The cellular uptake, cytotoxicity, and in vitro therapeutic efficacy of PFC@PEPP-Fe were assessed using 4T1 breast cancer cells. In vivo bimodal imaging using fluorescence (FL) and ultrasound (US) was conducted after injection into 4T1 tumor-bearing balb/c nude mice. The synergistic anticancer effects of PFC@PEPP-Fe, combining CDT and PTT, were evaluated following 808 nm laser irradiation (1 W/cm²) for 5 min, with treatment outcomes monitored over a 14 days period. Results: Both in vitro and in vivo studies demonstrated that PFC@PEPP-Fe enables effective bimodal imaging and exhibits substantial anticancer efficacy through the synergistic effects of PTT and CDT. Near-infrared (NIR) laser irradiation increased the temperature, enhancing the release of O2 and the production of H2O2, which in turn amplified the CDT effect. The combination of PFC@PEPP-Fe administration and NIR laser significantly reduced tumor volume, slowed tumor growth, and improved survival in 4T1 tumor-bearing mice, confirming the strong anticancer activity due to the PTT/CDT synergy. Conclusions: As a multifunctional theranostic nanoparticle, PFC@PEPP-Fe not only enables cancer cell-specific US/FL bimodal imaging through the generation of microbubbles from its PFC core and fluorescent PEPP shells but also facilitates synergistic chemodynamic and photothermal therapeutic actions under NIR laser irradiation, which induces the self-supply of H2O2 and O2 within cancer cells.
Keywords: Fenton reaction; bimodal imaging; cancer treatment; perfluorocarbon; polyepinephrine; theranostics.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



Similar articles
-
Carrier-free nanoparticles for cancer theranostics with dual-mode magnetic resonance imaging/fluorescence imaging and combination photothermal and chemodynamic therapy.Int J Pharm. 2025 Feb 25;671:125285. doi: 10.1016/j.ijpharm.2025.125285. Epub 2025 Jan 27. Int J Pharm. 2025. PMID: 39880146
-
MnCO3-mineralized polydopamine nanoparticles as an activatable theranostic agent for dual-modality imaging-guided photothermal therapy of cancers.Theranostics. 2022 Sep 21;12(15):6762-6778. doi: 10.7150/thno.77060. eCollection 2022. Theranostics. 2022. PMID: 36185599 Free PMC article.
-
A light-controllable specific drug delivery nanoplatform for targeted bimodal imaging-guided photothermal/chemo synergistic cancer therapy.Acta Biomater. 2018 Oct 15;80:308-326. doi: 10.1016/j.actbio.2018.09.024. Epub 2018 Sep 19. Acta Biomater. 2018. PMID: 30240955
-
Nanoagent-Mediated Photothermal Therapy: From Delivery System Design to Synergistic Theranostic Applications.Int J Nanomedicine. 2025 May 29;20:6891-6927. doi: 10.2147/IJN.S522736. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40458746 Free PMC article. Review.
-
Applications of nanotheranostics in the second near-infrared window in bioimaging and cancer treatment.Nanoscale. 2024 Dec 5;16(47):21697-21730. doi: 10.1039/d4nr03058c. Nanoscale. 2024. PMID: 39508492 Review.
References
-
- Karges J. Clinical Development of Metal Complexes as Photosensitizers for Photodynamic Therapy of Cancer. Angew Chem Int Ed Engl. 2022;61:e202112236. - PubMed
-
- Lv Z, He S, Wang Y, Zhu X. Noble Metal Nanomaterials for NIR-Triggered Photothermal Therapy in Cancer. Adv Healthc Mater. 2021;10:e2001806. - PubMed
-
- Zhang Y, Chen PH, Li B, Guo H, Zhu J, Dang Z. et al. Comprehensively Optimizing Fenton Reaction Factors for Antitumor Chemodynamic Therapy by Charge-Reversal Theranostics. ACS Nano. 2023;17:16743–56. - PubMed
-
- Wang L, Ge K, Duan J, Du X, Zhou G, Ma L. et al. A double-gain theranostic nanoplatform based on self-supplying H2O2 nanocomposites for synergistic chemodynamic/gas therapy. J Colloid Interface Sci. 2024;654:774–84. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

