Advancing the clinical assessment of glomerular podocyte pathology in kidney biopsies via super-resolution microscopy and angiopoietin-like 4 staining
- PMID: 39776814
- PMCID: PMC11700855
- DOI: 10.7150/thno.101498
Advancing the clinical assessment of glomerular podocyte pathology in kidney biopsies via super-resolution microscopy and angiopoietin-like 4 staining
Abstract
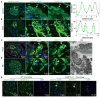
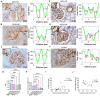
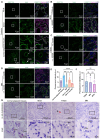
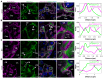
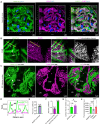
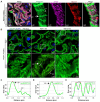
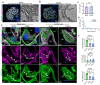
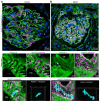
Rationale: The tertiary structure of normal podocytes prevents protein from leaking into the urine. However, observing the complexity of podocytes is challenging because of the scale differences in their three-dimensional structure and the close proximity between neighboring cells in space. In this study, we explored podocyte-secreted angiopoietin-like 4 (ANGPTL4) as a potential morphological marker via super-resolution microscopy (SRM). Methods and Results: Specimens from patients with minimal change disease (MCD), focal segmental glomerulosclerosis (FSGS), and membranous nephropathy (MN), along with normal controls, were analyzed via immunofluorescence and immunohistochemistry to determine the expression and localization of ANGPTL4, confirming its extensive presence in podocytes across both healthy and diseased conditions. Immunoelectron microscopy revealed that ANGPTL4 is distributed throughout the podocyte cell body, primary processes, and foot processes. Compared with conventional podocyte markers such as nephrin and synaptopodin, ANGPTL4 excels in depicting the three-dimensional structure of podocytes via SRM imaging. We then refined a protocol using tyramide signal amplification staining and confocal microscopy to uniformly enhance podocyte fluorescence, facilitating the clinical assessment of biopsies. In patients diagnosed with MCD and FSGS, measurements of slit diaphragm density, primary process width, and foot process width were taken after further co-staining with nephrin to identify patterns of podocyte morphological alterations. Distinctive patterns of foot process effacement were identified in MCD and FSGS patients, with FSGS patients showing more pronounced podocyte injury. Conclusions: ANGPTL4 serves as a reliable morphological marker for podocyte analysis, offering enhanced visualization of their three-dimensional structure and facilitating the identification of distinct pathological changes in nephrotic syndrome patients.
Keywords: 3D imaging; angiopoietin-like 4; podocytes; renal biopsy; super-resolution confocal microscopy.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

Similar articles
-
In situ evaluation of podocytes in patients with focal segmental glomerulosclerosis and minimal change disease.PLoS One. 2020 Nov 4;15(11):e0241745. doi: 10.1371/journal.pone.0241745. eCollection 2020. PLoS One. 2020. PMID: 33147279 Free PMC article.
-
Calcineurin inhibitors ameliorate PAN-induced podocyte injury through the NFAT-Angptl4 pathway.J Pathol. 2020 Nov;252(3):227-238. doi: 10.1002/path.5512. Epub 2020 Sep 8. J Pathol. 2020. PMID: 32686149
-
Dysregulation of podocyte phenotype in idiopathic collapsing glomerulopathy and HIV-associated nephropathy.Nephron. 2002 Jul;91(3):416-23. doi: 10.1159/000064281. Nephron. 2002. PMID: 12119471
-
Mechanisms and consequences of TGF-ß overexpression by podocytes in progressive podocyte disease.Cell Tissue Res. 2012 Jan;347(1):129-40. doi: 10.1007/s00441-011-1169-7. Epub 2011 May 4. Cell Tissue Res. 2012. PMID: 21541658 Free PMC article. Review.
-
[Structure and function of the glomerular filtration barrier].Pol Merkur Lekarski. 2005 Mar;18(105):317-20. Pol Merkur Lekarski. 2005. PMID: 15997642 Review. Polish.
Cited by
-
Hydrogen gas therapy: A promising approach for sepsis management post-burn injury by modulating inflammation, oxidative stress, and wound healing.Cytojournal. 2025 Apr 25;22:46. doi: 10.25259/Cytojournal_253_2024. eCollection 2025. Cytojournal. 2025. PMID: 40469704 Free PMC article.
References
-
- Brinkkoetter PT, Ising C, Benzing T. The role of the podocyte in albumin filtration. Nat Rev Nephrol. 2013;9:328–36. - PubMed
-
- Florian Grahammer CS, Tobias B Huber. The podocyte slit diaphragm-from a thin grey line to a complex signalling hub. Nat Rev Nephrol. 2013;9:587–98. - PubMed
-
- Nagata M. Podocyte injury and its consequences. Kidney Int. 2016;89:1221–30. - PubMed
-
- S O Bohman GJ, A B Bohlin, U Berg. Foot process fusion and glomerular filtration rate in minimal change nephrotic syndrome. Kidney Int. 1984;25:696–700. - PubMed
-
- Benzing T, Salant D. Insights into glomerular filtration and albuminuria. New Engl J Med. 2021;384:1437–46. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

