Engineered bio-functional material-based nerve guide conduits for optic nerve regeneration: a view from the cellular perspective, challenges and the future outlook
- PMID: 39776856
- PMCID: PMC11703557
- DOI: 10.1093/rb/rbae133
Engineered bio-functional material-based nerve guide conduits for optic nerve regeneration: a view from the cellular perspective, challenges and the future outlook
Abstract
Nerve injuries can be tantamount to severe impairment, standard treatment such as the use of autograft or surgery comes with complications and confers a shortened relief. The mechanism relevant to the regeneration of the optic nerve seems yet to be fully uncovered. The prevailing rate of vision loss as a result of direct or indirect insult on the optic nerve is alarming. Currently, the use of nerve guide conduits (NGC) to some extent has proven reliable especially in rodents and among the peripheral nervous system, a promising ground for regeneration and functional recovery, however in the optic nerve, this NGC function seems quite unfamous. The insufficient NGC application and the unabridged regeneration of the optic nerve could be a result of the limited information on cellular and molecular activities. This review seeks to tackle two major factors (i) the cellular and molecular activity involved in traumatic optic neuropathy and (ii) the NGC application for the optic nerve regeneration. The understanding of cellular and molecular concepts encompassed, ocular inflammation, extrinsic signaling and intrinsic signaling for axon growth, mobile zinc role, Ca2+ factor associated with the optic nerve, alternative therapies from nanotechnology based on the molecular information and finally the nanotechnological outlook encompassing applicable biomaterials and the use of NGC for regeneration. The challenges and future outlook regarding optic nerve regenerations are also discussed. Upon the many approaches used, the comprehensive role of the cellular and molecular mechanism may set grounds for the efficient application of the NGC for optic nerve regeneration.
Keywords: biomaterials; nerve guide conduits; optic nerve crush; optic neuropathy; regeneration.
© The Author(s) 2024. Published by Oxford University Press.
Figures



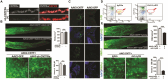

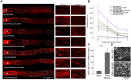
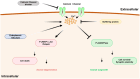
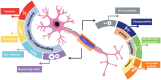



References
-
- Hench LL, Polak JM. Third-generation biomedical materials. Science 2002;295:1014–7. - PubMed
-
- Hench LL. Biomaterials. Science 1980;208:826–31. - PubMed
-
- Shirtliff V, Hench L. Bioactive materials for tissue engineering, regeneration and repair. J Mater Sci 2003;38:4697–707.
-
- Hench LL, Xynos ID, Polak JM. Bioactive glasses for in situ tissue regeneration. J Biomater Sci Polym Ed 2004;15:543–62. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

