CD28 shapes T cell receptor signaling by regulating Lck dynamics and ZAP70 activation
- PMID: 39776902
- PMCID: PMC11703918
- DOI: 10.3389/fimmu.2024.1503018
CD28 shapes T cell receptor signaling by regulating Lck dynamics and ZAP70 activation
Abstract
Introduction: T cell activation requires T cell receptor (TCR) engagement by its specific ligand. This interaction initiates a series of proximal events including tyrosine phosphorylation of the CD3 and TCRζ chains, recruitment, and activation of the protein tyrosine kinases Lck and ZAP70, followed by recruitment of adapter and signaling proteins. CD28 co-stimulation is also required to generate a functional immune response. Currently we lack a full understanding of the molecular mechanism of CD28 activation.
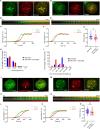
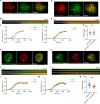
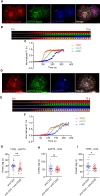
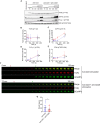
Methods: We employed TIRF microscopy to establish detailed spatial and kinetic relationships among these molecules in live Jurkat and murine primary T cells. We used anti-TCR (CD3) antibodies to trigger formation of TCR microclusters (MC), which are submicron-sized basic signaling units formed during T cell activation. Using this model, we aimed to delineate how the CD28 co-stimulatory signal alters the kinetics and molecular stoichiometry of TCR proximal signaling events, and how these effects could affect the immune response.
Results: Our results show that CD28 co-stimulation specifically accelerated recruitment of ZAP70 to the TCRζ chain in MCs and increased ZAP70 activation. CD28-mediated acceleration of ZAP70 recruitment was driven by enhanced Lck recruitment to the MCs. A greater spatial separation between active and inactive species of Lck was also observed in the MCs as a consequence of CD28 co-stimulation.
Conclusion: These results suggest that CD28 co- stimulation may lower the TCR activation threshold by enhancing the activated form of Lck in the TCR MCs.
Keywords: CD28 co-stimulation; Lck; T cell signaling; ZAP70; and kinetic proof-reading; microcluster.
Copyright © 2024 Raychaudhuri, Rangu, Ma, Alvinez, Tran, Pallikkuth, McIntire, Garvey, Yi and Samelson.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Update of
-
CD28 Shapes T Cell Receptor Signaling by Regulating ZAP70 Activation and Lck Dynamics.bioRxiv [Preprint]. 2024 Nov 22:2024.06.27.601067. doi: 10.1101/2024.06.27.601067. bioRxiv. 2024. Update in: Front Immunol. 2024 Dec 24;15:1503018. doi: 10.3389/fimmu.2024.1503018. PMID: 39372746 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

