Aspirin-triggered resolvin D1 modulates pulmonary and neurological inflammation in an IL-22 knock-out organic dust exposure mouse model
- PMID: 39776904
- PMCID: PMC11705093
- DOI: 10.3389/fimmu.2024.1495581
Aspirin-triggered resolvin D1 modulates pulmonary and neurological inflammation in an IL-22 knock-out organic dust exposure mouse model
Abstract
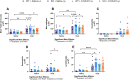
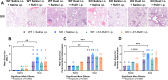
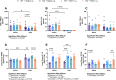
Agriculture dust contains many organic immunogenic compounds, and organic dust exposure is strongly associated with the development of immune-mediated chronic pulmonary diseases such as chronic obstructive pulmonary disease (COPD). Chronic organic dust exposure from agriculture sources induces chronic lung inflammatory diseases and organic dust exposure has recently been linked to an increased risk of developing dementia. The cytokine interleukin-22 (IL-22) has been established as an important mediator in the resolution and repair of lung tissues. The omega-3 fatty acid metabolite aspirin-triggered Resolvin D1 (AT-RvD1) has shown efficacy in modulating the immune response in both pulmonary and neurological inflammation but has not been explored as a therapeutic in organic dust exposure-induced neuroinflammation. Investigating the link between IL-22 and AT-RvD1 may help in developing effective therapies for these immune-mediated diseases. We aimed to investigate the link between organic dust exposure and neuroinflammation, the role of IL-22 in the pulmonary and neurological immune response to organic dust exposure, and the immune-modulating therapeutic applications of AT-RvD1 in an IL-22 knock-out mouse model of organic dust exposure. C57BL/6J (WT) and IL-22 knock-out (KO) mice were repetitively exposed to aqueous agriculture organic dust extract (DE) 5 days per week for 3 weeks (15 total instillations) and treated with AT-RvD1 either once per week (3 total injections) or 5 times per week (15 total injections) for 3 weeks and allowed to recover for 3 days. We observed a significant pulmonary and neurological immune response to DE characterized by the development of inducible bronchus associated lymphoid tissue in the lung and gliosis in the frontal areas of the brain. We also observed that IL-22 knock-out increased pulmonary and neurological inflammation severity. Animals exposed to DE and treated with AT-RvD1 displayed reduced lung pathology severity and gliosis. Our data demonstrate that DE exposure contributes to neurological inflammation and that IL-22 is crucial to effective tissue repair processes. Our data further suggest that AT-RvD1 may have potential as a novel therapeutic for organic dust exposure-induced, immune-mediated pulmonary and neurological inflammation, improving outcomes of those with these diseases.
Keywords: AT-RvD1; SPM; agriculture dust; aspirin-triggered resolvin D1; lung inflammation; neuroinflammation; omega-3 fatty acids.
Copyright © 2024 Threatt, White, Klepper, Brier, Dean, Ibarra, Harris, Jones, Wahl, Barahona, Oyewole, Pauly, Moreno and Nordgren.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer AU declared a past co-authorship with the author TN to the handling editor.
Figures





Similar articles
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Spectral immune cell profiling reveals modulations in immune cell response to repetitive inhaled organic dust exposure in a high omega-3 fatty acid mouse model.Lipids Health Dis. 2025 Jul 2;24(1):227. doi: 10.1186/s12944-025-02651-1. Lipids Health Dis. 2025. PMID: 40604979 Free PMC article.
-
Computer and mobile technology interventions for self-management in chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2017 May 23;5(5):CD011425. doi: 10.1002/14651858.CD011425.pub2. Cochrane Database Syst Rev. 2017. PMID: 28535331 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
References
-
- Centers for Disease Control and Prevention . Mortality in the United States National center for health statistics (2021). Available online at: https://www.cdc.gov/nchs/fastats/leading-causes-of-death.htm (Accessed September 4, 2024).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

