PGM3 insufficiency: a glycosylation disorder causing a notable T cell defect
- PMID: 39776909
- PMCID: PMC11703855
- DOI: 10.3389/fimmu.2024.1500381
PGM3 insufficiency: a glycosylation disorder causing a notable T cell defect
Abstract
Background: Hypomorphic mutations in the phosphoacetylglucosamine mutase 3 (PGM3) gene cause a glycosylation disorder that leads to immunodeficiency. It is often associated with recurrent infections and atopy. The exact etiology of this condition remains unclear.
Objective: This study aimed to characterize the phenotypes and immunological features associated with PGM3 insufficiency and investigate potential disease mechanisms.
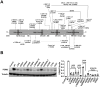
Methods: A systematic review of 44 published cases of PGM3 variants was performed, followed by T-cell phenotyping of two patients with PGM3 variants. A genotype-phenotypic severity study was conducted by comparing the residual PGM3 expression of the 12 reconstituted variants in human B cells. A PGM3 inhibitor was used to assess its effect on CD4+ T cell proliferation and differentiation.
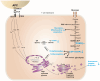
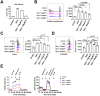
Results: Patients with PGM3 variants frequently presented with recurrent infections and atopy, accompanied by reduced naïve CD4+ T cell counts. A genotype-phenotype study showed that low levels of residual PGM3 expression are correlated with disease severity. Notably, inhibition of PGM3 activity impaired TCR-mediated CD4+ T cell proliferation and the synthesis of UDP-GlcNAc, complex N-glycans, O-GlcNAc, glycolytic stress, and mitochondrial respiration during proliferation in a dose-dependent manner. Partial loss of PGM3 activity was observed to preferentially enhance Th1 and Th2 differentiation, while attenuating Th17 and Treg differentiation, consistent with clinical observations.
Conclusion: PGM3 is a critical regulator of CD4+ T-cell proliferation and differentiation. These findings provide new insights into the diverse clinical manifestations and therapeutic development of PGM3 deficiency.
Keywords: CD4+ T cells; PGM3 insufficiency; UDP-GlcNAc; glycosylation; infections.
Copyright © 2024 Yang, Zerbato, Pessina, Brambilla, Andreani, Frey-Jakobs, Fliegauf, Barbouche, Zhang, Chiaradonna, Proietti, Du and Grimbacher.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Zhang Y, Yu X, Ichikawa M, Lyons JJ, Datta S, Lamborn IT, et al. Autosomal recessive phosphoglucomutase 3 (PGM3) mutations link glycosylation defects to atopy, immune deficiency, autoimmunity, and neurocognitive impairment. J Allergy Clin Immunol. (2014) 133:1400–1409.e1-5. doi: 10.1016/j.jaci.2014.02.013 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

