New insights into the management of homozygous familial hypercholesterolemia patients treated with lomitapide: a single-center experience
- PMID: 39777225
- PMCID: PMC11703714
- DOI: 10.3389/fendo.2024.1515846
New insights into the management of homozygous familial hypercholesterolemia patients treated with lomitapide: a single-center experience
Abstract
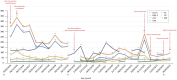
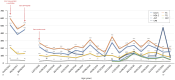
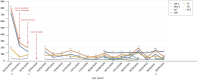
Familial hypercholesterolemia (FH) is a genetic disease, usually with onset during childhood, characterized by elevated blood LDL cholesterol levels and potentially associated with severe cardiovascular complications. Concerning mutated genes in FH, such as LDLR, a small subset of FH patients presents a homozygous genotype, resulting in homozygous FH (HoFH) disease with a generally aggressive phenotype. Besides statins, ezetimibe and PCSK9 inhibitors, lomitapide (an anti-ApoB therapy) was also approved in 2012-2013 as an adjunctive treatment for HoFH. Despite its clinical efficacy, lomitapide administration should be done with caution because of the possible occurrence of side effects, such as hepatosteatosis, increased blood transaminase levels and gastrointestinal symptoms, as well as the possible deleterious interactions with other drugs. In this context, we decided to report the main available evidence on the management and monitoring of HoFH patients treated with lomitapide and to accompany this literature review with a description of our clinical experience with a subset of six HoFH patients. In conclusion, this paper aims to address an important topic for HoFH-related clinical practice that, to our knowledge, is not yet formally regulated by proper national and/or international guidelines.
Keywords: HoFH; hypercholesterolemia; lomitapide; management; monitoring.
Copyright © 2024 Iannuzzo, Calcaterra, Gentile, Stanzione, de Ruberto, di Taranto, Cardiero, Fortunato and Minno.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures




Similar articles
-
Efficacy of Lomitapide in the Treatment of Familial Homozygous Hypercholesterolemia: Results of a Real-World Clinical Experience in Italy.Adv Ther. 2017 May;34(5):1200-1210. doi: 10.1007/s12325-017-0531-x. Epub 2017 Apr 21. Adv Ther. 2017. PMID: 28432645
-
Inhibition of hepatic microsomal triglyceride transfer protein - a novel therapeutic option for treatment of homozygous familial hypercholesterolemia.Vasc Health Risk Manag. 2014 May 6;10:263-70. doi: 10.2147/VHRM.S36641. eCollection 2014. Vasc Health Risk Manag. 2014. PMID: 24851052 Free PMC article. Review.
-
Lomitapide for the management of homozygous familial hypercholesterolemia.Rev Cardiovasc Med. 2014;15(2):109-18. doi: 10.3909/ricm0735. Rev Cardiovasc Med. 2014. PMID: 25051128 Review.
-
Lomitapide in homozygous familial hypercholesterolemia: cardiology perspective from a single-center experience.J Cardiovasc Med (Hagerstown). 2018 Mar;19(3):83-90. doi: 10.2459/JCM.0000000000000620. J Cardiovasc Med (Hagerstown). 2018. PMID: 29389816
-
Lomitapide for the treatment of pediatric homozygous familial hypercholesterolemia.Expert Opin Pharmacother. 2025 Aug;26(11-12):1289-1295. doi: 10.1080/14656566.2025.2545800. Epub 2025 Aug 19. Expert Opin Pharmacother. 2025. PMID: 40779345 Review.
Cited by
-
A Comprehensive Review of the Latest Approaches to Managing Hypercholesterolemia: A Comparative Analysis of Conventional and Novel Treatments: Part II.Pharmaceuticals (Basel). 2025 Aug 1;18(8):1150. doi: 10.3390/ph18081150. Pharmaceuticals (Basel). 2025. PMID: 40872541 Free PMC article. Review.
References
-
- Cuchel M, Meagher EA, Du Toit Theron H, Blom DJ, Marais AD, Hegele RA, et al. . Efficacy and safety of a microsomal triglyceride transfer protein inhibitor in patients with homozygous familial hypercholesterolaemia: a single-arm, open-label, phase 3 study. Lancet. (2013) 381:40–6. doi: 10.1016/S0140-6736(12)61731-0 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

