Assessing the Feasibility of Drone-Mediated Vaccine Delivery: An Exploratory Study
- PMID: 39777282
- PMCID: PMC11705479
- DOI: 10.1002/hsr2.70208
Assessing the Feasibility of Drone-Mediated Vaccine Delivery: An Exploratory Study
Abstract
Background and aims: In the past decade, unmanned aerial systems (UASs), commonly known as drones, have found applications not only in military and agriculture but also in the transportation of medical supplies.
Purpose: The present study was conducted to assess the practicality of utilizing drones as a mode for the delivery of vaccines to combat the challenges.
Study design: An exploratory study.
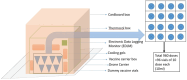
Methodology: Due to the COVID-19 pandemic restrictions and paucity of availability of rules and regulations related to drones in India in 2021, this study was conducted as a exploratory study for which number of regulatory approvals are obtained and it involves five drone missions within the premises of the Indian Institute of Technology (IIT), Kanpur, India on a confined airstrip of 3 km2 to transport simulated vaccine vials using a multi-rotor top-load UAS in the normal weather conditions in daylight where dummy vaccine vials (COVID-19) were packed with cool packs to maintain the temperature. Study was conducted to explore feasibility to carry vaccines through drones and any environmental impact on the vaccine vials while its transportation.
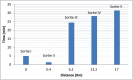
Results: The drones demonstrated a maximum flight endurance of 31 min while carrying a payload of up to 4.5 Kg, covering an aerial distance of 17 km at an average speed ranging at 12 m per second. Notably, the vaccine carrier box was able to maintain a recommended temperature of 3°C-4°C throughout the transportation process, and there is no impact of vibration on the physical integrity and leakage of the vaccine vials during flight.
Conclusions: These findings signify the potential for the drone-based medical supply deliveries across confined and controlled environment conditions. This study provides the insights that there is no environmental impact such as humidity, temperature, wind etc on the drone and no impact on vibrations on the physical integrity and leakage of the dummy vaccine vials. There were few regulatory barriers that required special approvals from concerned authorities. The study was not designed to assess for cost-effectiveness, also it was conducted in defined geography so all sorties were VLOS. Study has various limitations such as using simulated vaccine vials, regulatory barriers, operational barriers etc. Conducting the study in a controlled environment at IIT Kanpur limits generalizability. In spite of these limitations this study provides valuable insights and may explore a diverse environment that can help in strengthening health services especially in difficult terrains.
Keywords: COVID‐19; drones; immunization; unmanned aerial vehicle; vaccine; vaccine delivery.
© 2025 The Author(s). Health Science Reports published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Implementation of drone based delivery of medical supplies in North-East India: experiences, challenges and adopted strategies.Front Public Health. 2023 Jun 2;11:1128886. doi: 10.3389/fpubh.2023.1128886. eCollection 2023. Front Public Health. 2023. PMID: 37333530 Free PMC article.
-
U-Space and UTM Deployment as an Opportunity for More Complex UAV Operations Including UAV Medical Transport.J Intell Robot Syst. 2022;106(1):12. doi: 10.1007/s10846-022-01681-6. Epub 2022 Aug 24. J Intell Robot Syst. 2022. PMID: 36039343 Free PMC article.
-
Drone-based medical delivery in the extreme conditions of Himalayan region: a feasibility study.BMJ Public Health. 2024 Dec 10;2(2):e000894. doi: 10.1136/bmjph-2024-000894. eCollection 2024 Dec. BMJ Public Health. 2024. PMID: 40018596 Free PMC article.
-
Use of drones in clinical microbiology and infectious diseases: current status, challenges and barriers.Clin Microbiol Infect. 2020 Apr;26(4):425-430. doi: 10.1016/j.cmi.2019.09.014. Epub 2019 Sep 28. Clin Microbiol Infect. 2020. PMID: 31574337 Review.
-
Drone for medical products transportation in maternal healthcare: A systematic review and framework for future research.Medicine (Baltimore). 2020 Sep 4;99(36):e21967. doi: 10.1097/MD.0000000000021967. Medicine (Baltimore). 2020. PMID: 32899033 Free PMC article.
Cited by
-
Study on medical professionals' acceptance of and factors influencing drone delivery for medical supplies.Front Public Health. 2025 Jun 3;13:1571904. doi: 10.3389/fpubh.2025.1571904. eCollection 2025. Front Public Health. 2025. PMID: 40547476 Free PMC article.
-
Optimizing Vaccine Delivery With Drone Technology: Addressing Healthcare Disparities.Health Sci Rep. 2025 Mar 6;8(3):e70533. doi: 10.1002/hsr2.70533. eCollection 2025 Mar. Health Sci Rep. 2025. PMID: 40060297 Free PMC article. No abstract available.
References
-
- Michael O. T., Olusegun A., Paul O. A., et al., “The Use of UAV/Drones in the Optimization of Nigeria Vaccine Supply Chain,” International Journal of Scientific & Engineering Research 10, no. 10 (2019): 1273–1279, https://www.researchgate.net/profile/Timilehin-Omole/publication/3374868....
-
- Hii M., Courtney P., and Royall P., “An Evaluation of the Delivery of Medicines Using Drones,” Drones 3, no. 3 (September 2019): 52, 10.3390/drones3030052. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

