Panel containing three serum microRNAs: a promising biomarker for early detection of bladder cancer
- PMID: 39777350
- PMCID: PMC11703750
- DOI: 10.3389/fonc.2024.1470457
Panel containing three serum microRNAs: a promising biomarker for early detection of bladder cancer
Abstract
Background: Bladder cancer (BC) is a common tumor worldwide. Screening for BC currently lacks a highly efficient, non-invasive, and inexpensive method. Serum microRNA (miRNA), which is stable and commonly present, has the potential to serve as a novel marker for BC diagnosis.
Materials & methods: Based on a study involving 112 BC patients and 112 healthy subjects, we conducted this research in three phases to identify applicable microRNAs (miRNAs) in serum for BC diagnosis using quantitative reverse transcription polymerase chain reaction (qRT-PCR). A panel with optimal diagnostic value was developed. Additionally, we used bioinformatic analysis to explore the potential biological functions of the crucial miRNAs.
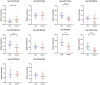
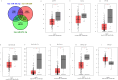
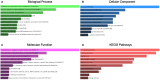
Results: The diagnostic panel consisted of miR-212-3p, miR-30c-5p, and miR-206, with an area under the curve (AUC) of 0.838, sensitivity of 83.33%, and specificity of 73.81%. Furthermore, ATF3, GJA1, JPH2, MVB12B, RUNX1T1, SLC8A1, SPATA6, and TPM3 may be potential target genes of these three miRNAs.
Conclusion: We developed a three-miRNA panel that could serve as a highly efficient and inexpensive biomarker for BC diagnosis and screening.
Keywords: MiR-212-3p; biomarker; bladder cancer; miR-206; miR-30c-5p; microRNA.
Copyright © 2024 Ge, Lin, Lu, Xia, Li, Li, Sun, Wen, Chen, Li, Li, Lin, Dong, Tao, Ji and Lai.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Identification of a three-miRNA panel in serum for bladder cancer diagnosis by a diagnostic test.Transl Cancer Res. 2022 May;11(5):1005-1016. doi: 10.21037/tcr-21-2611. Transl Cancer Res. 2022. PMID: 35706801 Free PMC article.
-
Testing the accuracy of a four serum microRNA panel for the detection of primary bladder cancer: a discovery and validation study.Biomarkers. 2024 Jul;29(5):276-284. doi: 10.1080/1354750X.2024.2358312. Epub 2024 May 30. Biomarkers. 2024. PMID: 38767408
-
A three-miRNA panel in serum: Serving as a novel diagnostic method for nasopharyngeal carcinoma.Int J Biol Markers. 2025 Jun;40(2):87-95. doi: 10.1177/03936155251329041. Epub 2025 Apr 15. Int J Biol Markers. 2025. PMID: 40232237
-
Expanding frontiers in liquid biopsy-discovery and validation of circulating biomarkers in renal cell carcinoma and bladder cancer.Int Rev Cell Mol Biol. 2025;391:135-197. doi: 10.1016/bs.ircmb.2024.08.005. Epub 2024 Sep 12. Int Rev Cell Mol Biol. 2025. PMID: 39939075 Review.
-
Diagnostic performance of microRNAs in the detection of heart failure with reduced or preserved ejection fraction: a systematic review and meta-analysis.Eur J Heart Fail. 2022 Dec;24(12):2212-2225. doi: 10.1002/ejhf.2700. Epub 2022 Oct 27. Eur J Heart Fail. 2022. PMID: 36161443 Free PMC article.
Cited by
-
Clinical application of the KangDuo-Surgical Robot-01 in distal gastrectomy for gastric cancer.Updates Surg. 2025 Jun;77(3):859-866. doi: 10.1007/s13304-025-02108-1. Epub 2025 Jan 20. Updates Surg. 2025. PMID: 39831930
References
LinkOut - more resources
Full Text Sources
Miscellaneous

