SPRINT Treatment Among Adults With Chronic Kidney Disease From 2 Large Health Care Systems
- PMID: 39777440
- PMCID: PMC11707627
- DOI: 10.1001/jamanetworkopen.2024.53458
SPRINT Treatment Among Adults With Chronic Kidney Disease From 2 Large Health Care Systems
Abstract
Importance: It is unclear whether the effects of intensive vs standard blood pressure (BP) targets seen in clinical trials generalize to patients with chronic kidney disease (CKD) encountered in everyday practice due to differences in the distribution of cardiovascular risk factors and coexisting conditions.
Objective: To evaluate whether the beneficial and adverse effects of intensive vs standard BP control observed in the Systolic Blood Pressure Intervention Trial (SPRINT) are transportable to a target population of adults with CKD in clinical practice.
Design, setting, and participants: This comparative effectiveness study identified 2 populations with CKD who met the eligibility criteria for SPRINT between January 1 and December 31, 2019, in the Veterans Health Administration (VHA) and Kaiser Permanente of Southern California (KPSC). Baseline covariate, treatment, and outcome data from SPRINT were combined with covariate data from these populations to estimate the treatment effects in the target population, applying models that estimated outcomes using distributions in the trial. Analysis was performed between May 2023 and October 2024.
Main outcomes and measures: The main outcomes were major cardiovascular events, all-cause death, cognitive impairment, CKD progression, and adverse events at 4 years.
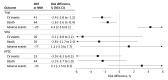
Results: A total of 85 938 patients (mean [SD] age, 75.7 [10.0] years; 81 628 [95.0%] male) from the VHA and 13 983 patients (mean [SD] age, 77.4 [9.6] years; 5371 [38.4%] male) from KPSC were included. Compared with 9361 SPRINT participants (mean [SD] age, 67.9 [9.4] years; 6029 [64.4%] male), these patients were older, had less prevalent cardiovascular disease, higher albuminuria, and used more statins. The associations of intensive vs standard BP control with major cardiovascular events, all-cause death, and adverse events were transportable from the trial to the VHA and KPSC populations; however, the trial's effects on cognitive and CKD outcomes were not transportable in 1 or both clinical populations. Intensive vs standard BP treatment was associated with lower absolute risks for major cardiovascular events at 4 years by 5.1% (95% CI, -9.8% to 3.2%) in the VHA population and 3.0% (95% CI, -6.3% to 0.3%) in the KPSC population and higher risks for adverse events by 1.3% (95% CI, -5.5% to 7.7%) in the VHA population and 3.1% (95% CI, -1.5% to 8.3%) in the KPSC population.
Conclusions and relevance: In this comparative effectiveness study, the reduction in fatal and nonfatal cardiovascular end points and the increase in adverse events observed in SPRINT were largely transportable to trial-eligible CKD populations from clinical practice, suggesting benefits of implementing intensive BP targets.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

