Interventions for quitting vaping
- PMID: 39777614
- PMCID: PMC11706636
- DOI: 10.1002/14651858.CD016058.pub2
Interventions for quitting vaping
Update in
-
Interventions for quitting vaping.Cochrane Database Syst Rev. 2024 May 21;5(5):CD016058. doi: 10.1002/14651858.CD016058. Cochrane Database Syst Rev. 2024. Update in: Cochrane Database Syst Rev. 2025 Nov 25;11:CD016058. doi: 10.1002/14651858.CD016058.pub3. PMID: 39908068 Free PMC article. Updated.
Abstract
Rationale: There is limited guidance on the best ways to stop using nicotine-containing vapes (otherwise known as e-cigarettes) and ensure long-term abstinence, whilst minimising the risk of tobacco smoking and other unintended consequences. Treatments could include pharmacological interventions, behavioural interventions, or both.
Objectives: To conduct a living systematic review assessing the benefits and harms of interventions to help people stop vaping compared to each other or to placebo or no intervention. To also assess how these interventions affect the use of combustible tobacco, and whether the effects vary based on participant characteristics.
Search methods: We searched the following databases from 1 January 2004 to 24 April 2024: CENTRAL; MEDLINE; Embase; PsycINFO; ClinicalTrials.gov (through CENTRAL); World Health Organization International Clinical Trials Registry Platform (through CENTRAL). We also searched the references of eligible studies and abstracts from the Society for Research on Nicotine and Tobacco 2024 conference, and contacted study authors.
Eligibility criteria: Randomised controlled trials (RCTs) recruiting people of any age using nicotine-containing vapes, regardless of tobacco smoking status. Studies had to test an intervention designed to support people to quit vaping, and plan to measure at least one of our outcomes.
Outcomes: Critical outcomes: vaping cessation; change in combustible tobacco use at six months or longer; number of participants reporting serious adverse events (SAEs) at one week or longer.
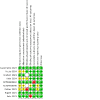
Risk of bias: We used the Cochrane RoB 1 tool to assess bias in the included studies.
Synthesis methods: We followed standard Cochrane methods for screening and data extraction. We grouped studies by comparisons and outcomes reported, and calculated individual study and pooled effects, as appropriate. We used random-effects Mantel-Haenszel methods to calculate risk ratios (RR) with 95% confidence intervals (CI) for dichotomous outcomes. We used random-effects inverse variance methods to calculate mean differences and 95% CI for continuous outcomes. We assessed the certainty of the evidence using the GRADE approach.
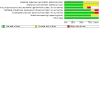
Included studies: Nine RCTs, representing 5209 participants motivated to stop using nicotine-containing vapes at baseline, are included. In six studies, participants were abstinent from smoking tobacco cigarettes at baseline, although most studies included some participants who had previously smoked. Eight studies included participants aged 18 or older, three included only young adults (18 to 24 years), and one included 13- to 17-year-olds only. We judged three studies at low risk, three at high risk, and three at unclear risk of bias.
Synthesis of results: Pharmacological interventions for quitting nicotine vaping Studies assessed combination nicotine replacement therapy (NRT), cytisine, and varenicline as pharmacological interventions for quitting vaping in comparison to placebo or no/minimal support (control). The point estimate for combination NRT indicated possible benefit, but the CI incorporated the possibility of no benefit and a potential benefit of control (very low-certainty evidence due to imprecision and risk of bias; RR 2.57, 95% CI 0.29 to 22.93; 1 study, 16 participants). The one study investigating cytisine did not report vaping cessation rates at six months or longer. Varenicline increased vaping cessation rates at six months, but the evidence was low certainty due to imprecision (RR 2.00, 95% CI 1.09 to 3.68; 1 study, 140 participants). Zero participants reported SAEs in the studies of combination NRT versus no/minimal support (1 study, 508 participants; low-certainty evidence due to imprecision) and cytisine versus placebo (1 study, 159 participants; low-certainty evidence due to imprecision). Three studies investigating varenicline measured the number of participants reporting SAEs. However, only one study reported an SAE (in the intervention arm); therefore, the effect estimate was calculated based on that single study (RR 2.60, 95% CI 0.11 to 62.16; 95 participants; low-certainty evidence due to imprecision). Behavioural interventions for quitting nicotine vaping Studies assessed reducing nicotine concentration and vaping behaviour (1 study) and text message-based interventions (3 studies) as behavioural interventions for stopping vaping in comparison to no/minimal support (control). In one study, the point estimate suggested nicotine/vaping reduction increased vaping cessation compared to minimal support at six-month follow-up, but the CI incorporated the possibility of no intervention effect and higher cessation rates in the control arm (RR 3.38, 95% CI 0.43 to 26.30; 17 participants; very low-certainty due to imprecision and risk of bias). There was low-certainty evidence (downgraded two levels due to indirectness) that text message-based interventions may have increased vaping cessation rates compared to control in 13- to 24-year-olds (RR 1.32, 95% CI 1.19 to 1.47; I2 = 0%; 2 studies, 4091 participants). The one study investigating nicotine/vaping behaviour reduction did not report on SAEs. One of the studies investigating text message-based interventions did report on SAEs; however, zero events were reported in both study arms (508 participants; low-certainty evidence due to imprecision). No studies reported change in combustible tobacco smoking at six-month follow-up or longer.
Authors' conclusions: There is low-certainty evidence that text message-based interventions designed to help people stop nicotine vaping may help more youth and young adults to successfully stop than no/minimal support, and low-certainty evidence that varenicline may also help people quit vaping. Data exploring the effectiveness of combination NRT, cytisine, and nicotine/vaping behaviour reduction are inconclusive due to risk of bias and imprecision. Most studies that measured SAEs reported none; however, more data are needed to draw clear conclusions. Of note, data from studies investigating these interventions for quitting smoking have not demonstrated serious concerns about SAEs. No studies assessed the change in combustible tobacco smoking, including relapse to or uptake of tobacco smoking, at six-month follow-up or longer. It is important that future studies measure this so the complete risk profile of relevant interventions can be considered. We identified 20 ongoing RCTs. Their incorporation into the evidence base and the continued identification of new studies is imperative to inform clinical and policy guidance on the best ways to stop vaping. Therefore, we will continue to update this review as a living systematic review by running searches monthly and updating the review when relevant new evidence that will strengthen or change our conclusions emerges.
Funding: Cancer Research UK (PRCPJT-Nov22/100012). National Institute of Health Research (NIHR206123) REGISTRATION: Protocol available via DOI: 10.1002/14651858.CD016058.
Copyright © 2025 The Authors. Cochrane Database of Systematic Reviews published by John Wiley & Sons, Ltd. on behalf of The Cochrane Collaboration.
Conflict of interest statement
ARB's work on this review has been supported by Cancer Research UK Project Award funding. This is not deemed a conflict of interest.
NL has received payment for lectures on systematic review methodology (Oxford University Hospitals NHS Foundation Trust), and has been an applicant and principal investigator on project funding to carry out research in the area of tobacco control from the NIHR Evidence Synthesis programme, Cancer Research UK (charity), Clarion Futures (charity), Oxfordshire County Council and the NIHR Oxfordshire and Thames Valley ARC, and Greater Manchester NHS Integrated Care. None of this is deemed a conflict of interest.
JLB was employed by the University of Oxford to work as a Managing Editor and Information Specialist for the Cochrane Tobacco Addiction Group before becoming an author on this review. During this time, he was involved in the editorial processing of the review. He is now an Editor for Cochrane. Since becoming an author, he has not been involved in the editorial process for this review. Core infrastructure funding for the Cochrane Tobacco Addiction Group was provided by the National Institute for Health and Care Research (NIHR) to the University of Oxford.
CN has received an honorarium from Vox Media for filming a 'nicotine explainer' on the role of nicotine in addiction. This is not deemed a conflict of interest. CN is a member of the advisory council for 'Action on Smoking and Health (ASH)'. CN is lead author of the Cochrane review of incentives for smoking cessation.
TT has no known conflicts of interest. TT is a Cochrane editor, but was not involved in the editorial process for this review.
NAR has received royalties from UpToDate, Inc., for chapters on electronic cigarettes and occasional fees from academic hospitals or professional medical societies for lectures on smoking cessation that include discussion of electronic cigarettes. NAR was a member of the committee that produced the 2018 National Academies of Science, Engineering, and Medicine's Consensus Study Report on the Public Health Benefits of E‐cigarettes. She was unpaid for this work. NAR is employed by Massachusetts General Hospital (MGH). Outside the topic of e‐cigarettes, NAR previously consulted for Achieve LifeSciences, which is developing an investigational smoking cessation medication for US Food and Drug Administration (FDA) approval (cytisine), and her institution (MGH) receives a grant from the company as a site for a clinical trial testing the safety and efficacy of cytisine for smoking and vaping cessation. NAR holds grants from the National Institutes of Health (NIH) for other research work.
TRF receives funding from the NIHR Community Healthcare MedTech and In Vitro Diagnostics Co‐operative at Oxford Health NHS Foundation Trust (MIC‐2016‐018) and the NIHR Applied Research Collaboration Oxford and Thames Valley at Oxford Health NHS Foundation Trust. The views expressed are those of the authors and not necessarily those of the National Health Service (NHS), the National Institute for Health and Care Research (NIHR), or the Department of Health and Social Care.
LD has received grants from the National Institute of Health Research (NIHR) Public Health Research (PHR) September 2021 to August 2024, and the Medical Research Council (MRC) Public Health Intervention Development Scheme (PHIND) March 2021 to May 2022. LD has received consulting fees from and acted as an Advisory Board Member for the development of smoking cessation resources for Nicorette UK, Johnson & Johnson. LD's other/non‐financial interest: interviewee for TV.net Latvia: one‐hour programme on e‐cigarettes 23 February 2023. LD has an affiliation to the following organisations that have a declared opinion or position on the topic: Action on Smoking and Health (ASH) (Advisory Council Member); Drug Science (Member of the Scientific Committee). Other: Invited Speaker: All Party Parliamentary Group (APPG) on vaping 19 April 2023.
RB holds a National Institute for Health Research (NIHR) grant, but this did not directly fund this current work.
ADW's work on this review has been supported by Cancer Research UK Project Award funding. This is not deemed a conflict of interest.
LB is funded through the NIHR Policy Research Unit in Addictions. The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
MC's work is supported by Cancer Research UK (charity). She is a Cochrane Proposal Editor, but was not involved in the editorial process for this review. None of this was deemed a conflict of interest.
ES is funded through the NIHR Policy Research Unit in Addictions. The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
JHB has received payment for consulting from the Truth Initiative, and payment for board membership from the US Food and Drug Administration. She has received funding (to her institution) to carry out research in the area of tobacco control from the NIHR Evidence Synthesis programme, Cancer Research UK (charity), and the National Institutes of Health and US Food and Drug Administration. She is a member of Health Canada's Scientific Advisory Board for Vaping Products. None of this is deemed a conflict of interest.
Figures
References
-
- ADDICTO:0000212. E-cigarette, Addiction Ontology Vocabulary Interface. https://addictovocab.org/ADDICTO:0000212 (accessed 1 August 2024).
-
- ADDICTO:0000232. E-liquid, Addiction Ontology Vocabulary Interface. https://addictovocab.org/ADDICTO:0000232 (accessed 1 August 2024).
-
- NHS. Vaping to quit smoking. www.nhs.uk/better-health/quit-smoking/vaping-to-quit-smoking/ (accessed 18 September 2023).
-
- Govuk. Smokers urged to swap cigarettes for vapes in world first scheme. https://www.gov.uk/government/news/smokers-urged-to-swap-cigarettes-for-... 2023 (accessed 18 September 2023).
-
- Ministry of Health New Zealand. The New Zealand guidelines for helping people to stop smoking. 2021 Update. https://www.health.govt.nz/system/files/documents/publications/the-new-z... (accessed 18 September 2023).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous