Prophylactic Peanut Allergen Ara h 6 Sublingual Immunotherapy Drives Expansion of FoxP3 +Helios- Regulatory T Cells in the Absence of Allergen-Specific IgA
- PMID: 39777617
- PMCID: PMC11799393
- DOI: 10.1111/imm.13883
Prophylactic Peanut Allergen Ara h 6 Sublingual Immunotherapy Drives Expansion of FoxP3 +Helios- Regulatory T Cells in the Absence of Allergen-Specific IgA
Abstract
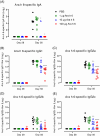
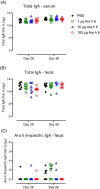
Insights into the underlying immunological mechanisms of prophylactic sublingual immunotherapy (SLIT) may support the development of new strategies for improved prevention and treatment of food allergy. Here, we investigated the humoral, regulatory and sublingual tissue immune response to prophylactic SLIT administration of a single purified peanut allergen in Brown Norway (BN) rats. BN rats received daily sublingual administration of peanut allergen Ara h 6 for three weeks. Suppression of sensitisation was evaluated by subsequent intraperitoneal administration of Ara h 6. Ara h 6-specific IgE, IgA, IgG1 and IgG2a-c levels were measured in serum. The frequency of regulatory T (Treg) cells was analysed using flow cytometry. The sublingual tissue response to Ara h 6 was analysed by transcriptional profiling using mRNA-sequencing. Ara h 6 SLIT protected rats from subsequent sensitisation without inducing a detectable humoral immune response (Ara h 6-specific IgE, IgA, IgG1 and IgG2a-c) in serum. SLIT furthermore promoted the relative expansion of induced Helios- Treg cells within the conventional CD4+CD25+FoxP3+ Treg population in sublingual draining lymph nodes and blood. In conclusion, prophylactic Ara h 6 SLIT drives the relative expansion of induced Helios- Treg cells in the absence of Ara h 6-specific IgA highlighting a potential novel IgA-independent Treg-related immune response at the sublingual mucosal site.
Keywords: immune response; peanut allergy; prophylaxis; sublingual immunotherapy; tissue response.
© 2025 The Author(s). Immunology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Utility of component analyses in subjects undergoing sublingual immunotherapy for peanut allergy.Clin Exp Allergy. 2016 Feb;46(2):347-53. doi: 10.1111/cea.12635. Clin Exp Allergy. 2016. PMID: 26362760 Free PMC article. Clinical Trial.
-
Enhanced Prophylactic and Therapeutic Effects of Polylysine-Modified Ara h 2 DNA Vaccine in a Mouse Model of Peanut Allergy.Int Arch Allergy Immunol. 2016;171(3-4):241-250. doi: 10.1159/000453264. Epub 2017 Jan 4. Int Arch Allergy Immunol. 2016. PMID: 28049187
-
Analysis of cytokine production by peanut-reactive T cells identifies residual Th2 effectors in highly allergic children who received peanut oral immunotherapy.Clin Exp Allergy. 2015 Jul;45(7):1201-13. doi: 10.1111/cea.12537. Clin Exp Allergy. 2015. PMID: 25823600 Free PMC article.
-
Redefining the major peanut allergens.Immunol Res. 2013 Mar;55(1-3):125-34. doi: 10.1007/s12026-012-8355-x. Immunol Res. 2013. PMID: 22948807 Free PMC article. Review.
-
Current Trend in Immunotherapy for Peanut Allergy.Int Rev Immunol. 2018;37(6):279-290. doi: 10.1080/08830185.2018.1509967. Epub 2019 Jan 13. Int Rev Immunol. 2018. PMID: 30638084 Review.
References
-
- Sicherer S. H. and Sampson H. A., “Food Allergy: A Review and Update on Epidemiology, Pathogenesis, Diagnosis, Prevention, and Management,” Journal of Allergy and Clinical Immunology 141 (2018): 41–58. - PubMed
-
- Greenhawt M., “Food Allergy Quality of Life and Living With Food Allergy,” Current Opinion in Allergy and Clinical Immunology 16 (2016): 284–290. - PubMed
-
- Nurmatov U., Dhami S., Arasi S., et al., “Allergen Immunotherapy for Ige‐Mediated Food Allergy: A Systematic Review and Meta‐Analysis. Allergy: European,” Journal of Allergy and Clinical Immunology 72 (2017): 1133–1147. - PubMed
-
- Pajno G. B., Fernandez‐Rivas M., Arasi S., et al., “EAACI Guidelines on Allergen Immunotherapy: IgE‐Mediated Food Allergy,” Allergy 73 (2018): 799–815. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

